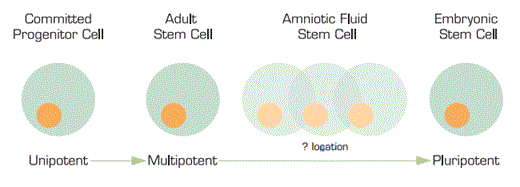
We are all fully aware of the stem cell research bill previously vetoed by President Bush. A stem cell bill has now been re-approved by the House of Representatives and is being considered in the Senate to expand the use and availability of embryonic stem cells for research due to their ability for pluripotentiality. We are all aware that, rarely, multipotential or maybe even pluripotential stem cells have been isolated from cultured marrow cells and from spermatogonial cells of the testis. Now, researchers from Wake Forest University have isolated stem cells from amniotic fluid that are capable of giving rise to a number of different cell types. The big question is — where on the continuum do they fit? Or, in other words, how “plastic” are they? They clearly are not as rigid as multi-potential cells. But are they just as flexible as embryonic stem cells? And, if so, are they a replacement for the hotly politically-contested embryonic stem cells?
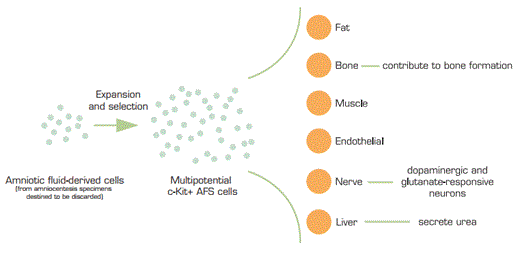
Amniotic fluid has long been known to contain a very heterogeneous population of cells, which are derived from origins of fetal tissues as well as the amnion. The amnion arises from the epiblast of the differentiating blastocyst and forms a membrane which surrounds the fetus. The “amniotic cavity” is maintained through the entirety of gestation and is created during the second week of development, between the embryonic ectoderm and mesoderm. Utilizing back-up amniocentesis specimens that were destined to be discarded, De Coppi and colleagues harvested cells from these confluent back-up human amniocentesis cultures. The cells were either expanded once or immediately subjected to immunoselection with CD117 (c-Kit). These c-Kit + amniotic fluid stem (AFS) cells represent about 1 percent of cells routinely found in amniotic fluid. The investigators passaged the cell lines for over 250 population doublings with retention of long telomeres and normal karyotypes. They state that this mark far exceeds the “Hayflick limit” of about 50 population doublings for many post-embryonic cells, a characteristic generally attributed to progressive shortening of telomeres. The AFS cell lines are not feeder-cell dependent and very well might be able to be produced in mass quantities.
The investigators further demonstrated that these AFS cells could give rise to a number of different cell types, including a propensity for adipogenic, osteogenic, myogenic, endothelial, neurogenic, and hepatic cell generation. Evidence that the AFS cells truly gave rise to functional cell types of many lineages included differentiation to: (1) nestin-positive neural stem cells maturing into dopaminergic and glutamate-responsive neurons; (2) putative hepatocytes, secreting urea and expressing albumin, a-fetoprotein, hepatocyte nuclear factor, and growth factor; and (3) functional osteoblasts that produce bone-like material when embedded in collagen scaffolds and grafted in immunodeficient mice.
In Brief
So, in many ways, cloned AFS cells were proven to be broadly multipotent and maybe even approaching the pluripotentiality of embryonic stem cells. But, importantly, none of the four AFS cell lines treated formed teratocarcinomas when implanted in vivo. Teratocarcinomas can be seen deriving from embryonic stem cells but are not seen from normal somatic stem cells. This potentially makes them safer than ES cells, at least from the viewpoint of the FDA, but does it also suggest a limitation and that maybe they do not have as much plasticity as embryonic stem cells? This question can only be answered by further study.
But will these important results and findings regarding AFS cells be misunderstood and misused in the contentious political debate over stem cells? A similar scenario has already arisen over the reprogramming of adult stem cells. Harvard University researchers Kevin Eggan, Chad Cowan, and Douglas A. Melton were quoted in the January 22, 2007, issue of The Washington Post as saying, “We are surprised to see our work on reprogramming adult stem cells used to support arguments that research involving human embryonic stem cells is unnecessary. On the contrary, we assert that human embryonic stem cells hold great promise to find new treatments and cures for diseases...”
Competing Interests
Dr. Emanuel indicated no relevant conflicts of interest.