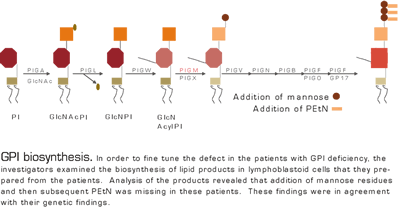
Somatic cell mutation of the gene that encodes PIGA results in the loss of formation of GPI-anchored proteins and is associated with PNH. In this paper, the investigators describe an autosomal recessive disease that results in GPI deficiency. In uncovering the etiology of childhood thrombosis and seizures in two unrelated families (but each consanguineous), a HAM test was found to be positive. GPI expression was reduced in both patients in some but not all hematopoietic cells. By homozygosity mapping and SNP analysis of the two families, they closed in on the PIGM gene as the possible culprit. Cell lines were isolated from the affected patients and their parents and radiolabeled glycolipids were isolated and analyzed. The products reached the Gln acyl PI stage (Figure), but the cell lines failed to add additional mannose and PEtN residues — implicating either PIGM or PIGX as the defective step. The combination of genetic information with the biochemical findings pointed to PIGM as the mutated gene.
In Brief
The analysis put forth by this group to explain the clinical course of two families with children who presented with thrombosis at a young age is not only an admirable piece of detective work but also a lesson in the application of biochemical insight into their genetic findings. Although it would be attractive to place the defect that the investigators uncovered in the context of more general clinical application, the thoroughness of their studies (which enabled them to discern a cellular defect) is the major take-home lesson from this work. The mutation is not in the expressed enzymatic portion of the gene, but rather in the upstream promoter that binds the transcription factor Sp-1. In explaining why a gene, PIGM, that is important in development, may lead to only mild neurological defects, acetylation status of the PIGM locus is suggested to play a role in promoting sufficient biosynthesis of the PIGM product to overcome the defect in Sp-1 binding. Alternatively, other promoters were detected (e.g., GATA) that may induce expression in a more tissue-restricted manner. Since these enzymatic pathways play a critical role in development across a number of tissues, it is difficult to surmise whether other defects will be uncovered to explain some similar thrombotic complications or whether this enzymatic step is at the threshold of sustaining development but still yields clinically significant findings when affected.
Competing Interests
Dr. Petruzzelli indicated no relevant conflicts of interest.