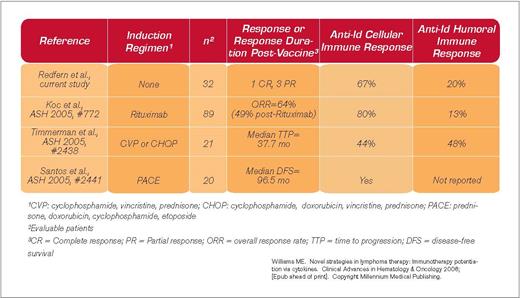
Each B-cell non-Hodgkin lymphoma (NHL) expresses a unique surface immunoglobulin that may serve as a target for immunotherapy. In this paper, Redfern and colleagues report the findings of a phase II trial wherein lymph node biopsies obtained from patients with relapsed indolent lymphoma were utilized for the isolation and cloning of the unique immunoglobulin “idiotype” (Id) sequences. These Id proteins were conjugated to KLH as an immunostimulant and administered subcutaneously on day 1 of each treatment, with injections of the cytokine GM-CSF at the vaccine site on days 1-4. Vaccine was administered monthly for six months, with booster doses every other month for the next year and then every three months for patients without disease progression. This trial, unlike most other vaccine studies, did not utilize induction chemotherapy or rituximab therapy prior to vaccination and thus provides insight as to single-agent activity and the ability to generate vaccine-specific humoral and cellular immune responses in these relapsed NHL patients. Patient-specific Id/KLH vaccines were generated for each of the 40 eligible patients, although eight patients did not receive vaccine due to disease progression or initiation of other therapy prior to vaccine generation. Of the 32 patients who received at least one dose of vaccine, one complete and three partial remissions were noted; the median time to response was 5.9 months. An additional 21 patients were reported to have stable disease. The median time to progression for all patients was 13.5 months, and at least 28 months for the four responders with ongoing response at 44+ months for the CR patient. The most common side effect of treatment was transient mild or moderate injection site reaction. Among the subset of patients assessed for immune response, 80 percent developed a T-cell and 89 percent a humoral anti-KLH response, with 67 percent and 20 percent developing T-cell or humoral anti-Id responses, respectively.
In Brief
Lymphoma Id vaccine therapy was originally developed by Levy and colleagues at Stanford University, who showed durable clinical responses in a subset of NHL patients. Although the original techniques for creation of these personalized vaccines was labor-intensive and could take six months or more, current recombinant technologies have high success rates for generating the Id proteins while shortening the time required for manufacture to only two to three months. Preliminary results of phase II Id vaccine trials recently have been presented (Table), and two multicenter phase III trials recently completed accrual with a third nearing completion. The results of the present study confirm the ability to generate Id vaccines for the majority of patients with relapsed indolent NHL. Furthermore, the vaccines are well tolerated and may lead to clinical responses, and both cellular and humoral immune responses can be generated. Weng, Czerwinski, and Levy recently summarized the Stanford experience for 180 follicular lymphoma patients treated with Id vaccination between 1988 and 2002; 36 percent developed a humoral and 24 percent a cellular immune response1 . The development of a cellular response was enhanced when either dendritic cells or GM-CSF were employed as an adjuvant for vaccination. Additional approaches to enhance therapeutic efficacy as well as cellular and humoral immune responses are currently being pursued. The results of the multicenter phase III Id vaccine studies noted above are awaited with interest to determine their ability to improve long-term disease control for indolent lymphomas.
References
Competing Interests
Dr. Williams indicated no relevant conflicts of interest.