In Brief
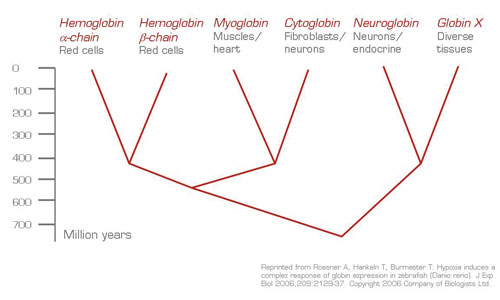
Since man is not a carp, do these elegant discoveries have any meaning for humans? The functional details, such as oxygen-binding properties, the presence or absence of Bohr effects on these diverse globins, their presence in different tissues and different species2 , and the subtleties of hypoxia regulation, of these genes await elucidation. Nevertheless, there is growing evidence that the newly discovered globins are also present in mammals and found in unorthodox places such as rectal smooth muscle cells, prostate, lungs, the brain, and endocrine organs3 . Their role in oxygen delivery, protection against nitric oxide, oxygen radical toxicity, adaptation to exercise, and hypoxia is also becoming established2,3 . One can also ask if the yet-to-be-explained neurotoxicity and failure to thrive that is characteristic of type 2 congenital methemoglobinemia4 may be caused by failure to keep the heme iron in these globins in ferrous state by ubiquitous deficiency of cytochrome b5 reductase. Clearly, we will soon learn more about the importance of these newly discovered globins; hematologists will benefit from Dr. Thorsten Burmester's upcoming lecture, Neuroglobin — Fresh Blood for the Globin Family, at the Red Cell Scientific Committee session at this year's ASH meeting.
References
Competing Interests
Dr. Prchal indicated no relevant conflicts of interest.