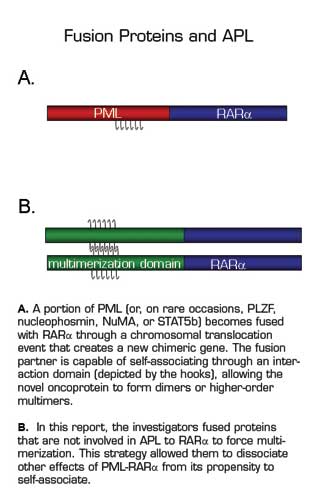
On the surface, the molecular cause of acute promyelocytic leukemia (APL) appears to be remarkably simple - APL patients carry an aberrant chromosomal translocation that disrupts the chromosome 17 gene encoding a retinoic acid-binding transcription factor, RARα. Almost always, a unique translocation [t(15;17)] leads to the production of a fusion oncoprotein made up of the first part of PML, an unrelated protein, and the last part of RARα (Figure 1A). In the sixteen years since RARα fusion proteins were first described in APL, investigators have tried to answer three questions: Why is RARα always altered? How do the translocations change a normal transcription factor into an oncoprotein? How do pharmaceutical doses of all-trans-retinoic acid (ATRA) overcome the leukemic process? In this report, Sternsdorf and colleagues provide partial answers, following up on many previous studies. They considered what PML has in common with the other proteins that have occasionally been found fused to RARα in APL - not much, other than the ability to self-associate. They asked whether other fusion partners engineered to make RARα multimerize (Figure 1B) might have similar properties. They showed that multimerization makes RARα act as a more potent transcriptional regulator of an expanded repertoire of target genes in vitro. However, when the novel multimerizing fusion proteins were expressed in mice, only a small fraction of the animals developed leukemia after a long latency period, suggesting that at least one other genetic event was necessary. They showed that leukemia could be potentiated in mice that also expressed an activated form of the βcommonsubunit of cytokine receptors, thus providing a second hit. They concluded that multimerization was important, but that the PML-RARα fusion protein must also have new, unknown properties that make it an oncoprotein. It cannot simply be that fusion to RARα prevents PML from doing its normal job, because forced RARα multimerization caused leukemia at the same low frequency in mice lacking PML altogether.
RARα , acting with its normal partner RXR, clearly has a special role as a transcriptional regulator of the myeloid development program. But there can be too much of a good thing. Forcing RARα to self-aggregate seems to make it better at shutting off transcription of its normal target genes and probably other genes as well, thus perturbing normal myeloid differentiation. This happens whether the fusion involves PML or an irrelevant protein that causes RARα to form multimers. It suggests that higher doses of ATRA, a molecule that normally modulates RARα activity, are required to overcome the stronger effects of RARα multimers. But the new protein created by fusion of PML to RARα must have at least one other, as yet unexplained, function that makes cells malignant. There are two important messages from this work. First, a single genetic event, the translocation that fuses PML to RARα, can have complex sequelae - not just multimerization of RARα, but also a new activity that has not yet been elucidated. Second, as the authors point out, it seems likely that drugs could be designed to interfere with RARα multimerization, providing a new option for treating APL.
Competing Interests
Dr. Andrews indicated no relevant conflicts of interest.