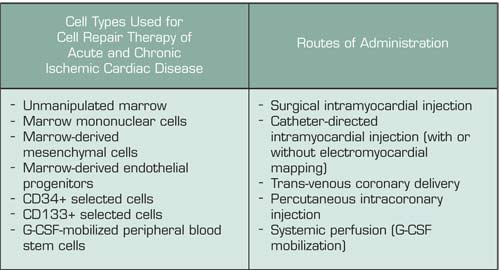
A great deal of excitement was generated five years ago when murine studies demonstrated that purified hematopoietic stem cells or cytokine-mobilized peripheral blood stem cells (PBSC) could engraft into ischemic myocardial tissue, regenerate functional myocardium, and thereby limit injury after acute myocardial infarction (MI). Phase I and I/II human studies quickly followed, with post-MI patients or patients with chronic ischemic heart disease receiving autologous progenitor cells isolated by various means and administered by a variety of different approaches (Table). Early trials with marrow-derived cells delivered by intracoronary infusion reported significant improvements in global left ventricular function and in regional perfusion and contractile function downstream of the infused vessel, suggesting a direct local effect of the cells and/or their trophic factors. The potential benefits of G-CSF administration after acute MI have been studied in two contexts: (1) as a means to collect high numbers of PBSC (by apheresis) for subsequent intracoronary infusion, and (2) to continuously perfuse the ischemic myocardium with a high level of PBSC to facilitate homing and engraftment of the cell types necessary for myocardial repair. In vitro studies also suggest that G-CSF can directly enhance cardiomyocyte survival, induce proliferation, and stimulate the release of proangiogenic mediators. Three phase I/II studies demonstrated the feasibility and relative safety of G-CSF when given for four to 10 days after acute MI, and each reported significant improvements in left ventricular function at three to six months after G-CSF. However, those trials were limited by lack of appropriate randomization, placebo controls, double-blind design, and/or adequate patient numbers.
In this paper, Zohlnhöfer et al. used a randomized double-blind, placebo-controlled design to assess the safety and benefit of G-CSF after an ST-segment elevation acute MI following successful reperfusion by percutaneous coronary stent placement. Fifty-six patients received G-CSF at 10 μg/kg/day for five days, starting five days after the MI, and 58 received placebo. G-CSF successfully mobilized progenitors, with a mean peak peripheral blood CD34+ cell count of 72/μL on day 5, and no major adverse events occurred. Blood CD34+ cell counts in the placebo group were also increased above baseline and ranged from 5 - 9/μL. Both groups showed a similar decrease in left ventricular infarct size at four and six months post-MI and a similar modest increase in left ventricular ejection fraction, with no significant difference between placebo and G-CSF. The rates of angiographic restenosis were also the same in both groups, suggesting that G-CSF was not prothrombotic in this setting.
In Brief
This study illustrates the importance of a carefully designed, randomized clinical trial to address the benefit of a therapeutic intervention. Of note, a recently completed randomized double-blind, placebo-controlled trial from Denmark also found no benefit of G-CSF when given for six days after acute MI. Together, these observations suggest that the previous encouraging data reflected inadequate study design or critical differences in technical or biological variables. A number of fundamental issues need to be addressed including the optimal number, phenotype, and composition of the stem cell inoculum; the ideal timing of cell delivery; the most effective and safe route of administration; and the most relevant and specific end points. An additional consideration is that local paracrine factors, such as vascular endothelial growth factor-2, might significantly enhance the regenerative effect of CD34+ cells in ischemic myocardium. Thus, one might envision combination therapies using hematopoietic stem cells with systemic agents and/or specialized intracoronary stents that elute specific factors. Since cardiovascular disease is still the leading cause of death in the U.S. and Europe, and one million U.S. adults suffer an MI annually, continued aggressive pursuit of cell therapy approaches for acute and chronic ischemic heart disease appears justified. Despite the disappointing results of this trial, the collective evidence to date sustains the hope that the promise of regenerative medicine will first be realized for cardiovascular disease.
Competing Interests
Dr. Linenberger indicated no relevant conflicts of interest.