Clearly defining multiple myeloma (MM) patients with high-risk disease is essential for both designing appropriate treatment strategies and for ensuring interpretability and consistency in research studies. Recent trials such as MASTER, GMMG-CONCEPT, and OPTIMUM have underscored the importance of risk- and response-adapted therapy rather than a one-size-fits-all approach.1-3 These studies demonstrate that treatment intensity and duration should be guided by underlying disease biology and depth of response, highlighting the need for precise, up-to-date risk definitions. Over the years, characterizing myeloma risk has evolved from the Durie-Salmon Staging System to the International Staging System (ISS) to the Revised ISS (R-ISS) of 2015, which incorporated cytogenetic features — del(17p), t(4;14), and t(14;16). To better segregate the 60% of patients classified as intermediate risk by R-ISS, the European Myeloma Network developed the R2-ISS, adding chromosome 1q gain/amplification (amp) based on data from newly diagnosed, transplant-eligible patients enrolled in clinical trials.4
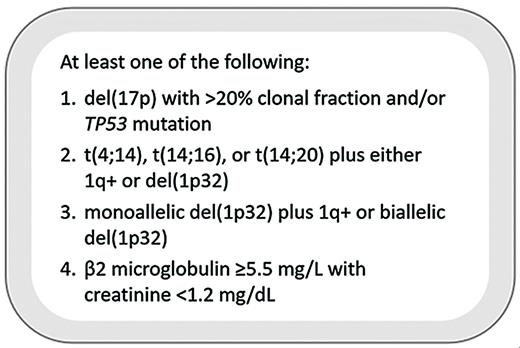
However, traditional staging systems have become outdated, failing to incorporate more recent data and losing their prognostic value in the era of novel triplets and quadruplets. Thus, in 2023, experts from the International Myeloma Society (IMS) and the International Myeloma Working Group (IMWG) convened to develop a data-driven set of consensus criteria describing the approximately 20% of MM patients with the highest-risk disease biology, with their findings published in 2025 (Figure).5
International Myeloma Society/International Myeloma Working Group high-risk criteria for multiple myeloma
International Myeloma Society/International Myeloma Working Group high-risk criteria for multiple myeloma
Presence of del(17p), the chromosome harboring TP53, is a well-recognized poor prognostic factor. The R-ISS, however, does not account for the proportion of myeloma cells harboring this abnormality, or the cancer clonal fraction (CCF). In previous studies, del(17p) positivity has been defined by CCF ranging from as low as 1% to as high as 60%.5 The consensus panel determined that a del(17p) CCF of 20% or greater separated patients with significantly worse progression-free survival (PFS) and overall survival (OS) and served as a useful threshold for defining high-risk patients. Furthermore, next-generation sequencing (NGS) is increasingly a part of molecular risk stratification in hematologic malignancies and can be particularly informative in TP53 characterization in MM. Only with NGS can it be determined if a patient with monoallelic loss of p53 has a concurrent TP53 mutation in the remaining allele. Multiple independent datasets (e.g., Myeloma XI, Multiple Myeloma Research Foundation [MMRF] CoMMpass, and Intergroupe Francophone du Myélome [IFM]) have confirmed that biallelic TP53 perturbation confers inferior PFS and OS.5
Additionally, translocations involving chromosome 14 have historically been considered high-risk, namely t(4;14), t(14;16), and the rarer t(14;20). However, current data suggest these translocations alone are not associated with worse outcomes. Rather, patients harboring one of these translocations along with either gain/amp(1q21) or del(1p32) had significantly worse PFS and OS based on the IFM dataset. Based on analyses of the MMRF CoMMpass and Myeloma XI datasets, patients with both aforementioned chromosome 1 abnormalities and those with biallelic del(1p32) (detected by loss of FAF1 or CDKN2C) also suffered from shorter PFS and OS, so these alterations were incorporated into the high-risk definition.
Although isolated 1q+ is also a poor prognostic marker, it is the most frequent cytogenetic abnormality and alone is not discerning enough to characterize high-risk disease. Also, amp(1q21) has not consistently conferred worse outcomes than gain(1q21), with Grupo Español de Mieloma and IFM data showing similar outcomes, and thus neither abnormality alone was considered high-risk.5
Finally, despite being a core component of ISS and R-ISS staging, the role of beta-2 microglobulin (β2M) as an independent prognostic factor in MM has been questioned. Since β2M increases with both tumor burden and renal impairment, it serves as a relatively nonspecific marker when considered in isolation. However, analyses of the CoMMpass and FORTE trials showed that elevated β2M (≥5.5 mg/L) in the context of preserved renal insufficiency (creatinine <1.2 mg/dL) was strongly associated with poor outcomes, comparable to molecularly defined high-risk disease, leading to its inclusion as a final high-risk feature.
In Brief
These updated, high-risk criteria incorporate important insights gained in recent years, including the importance of CCF, chromosome 1 abnormalities, and TP53 mutations, providing a more biologically meaningful definition of high-risk myeloma. Importantly, results of recent clinical trials reinforce the value of applying such criteria: GMMG-CONCEPT and OPTIMUM achieved impressive outcomes for high-risk patients using intensive post-transplant consolidation and multi-agent maintenance, while MASTER showed that standard-risk patients with sustained minimal residual disease (MRD) negativity may safely discontinue therapy.1-3 Furthermore, although fluorescence in situ hybridization has long been the standard for assessing genomic risk in MM, the recognition of TP53 mutations highlights the growing importance of NGS-based testing and may accelerate the shift toward targeted sequencing panels capable of concurrently detecting translocations, copy number variations, and mutations.
Future studies will need to also consider variables such as MYC translocations, NSD2 translocations, APOBEC mutational signatures, chromothripsis (reflected by copy number signatures), lambda light chain translocations, circulating plasma cells, large focal lesions on imaging, extramedullary disease, frailty, functional high risk, and inability to attain MRD negativity. Until then, the new data-driven criteria provide a more accurate and biologically relevant framework than prior staging systems such as the R-ISS and should be adopted as the standard definition of high-risk MM.
Disclosure Statement
Dr. Pan has received honoraria from Sanofi and research funding from Johnson & Johnson, and he reported consulting activity for Pfizer and Regeneron. Dr. Lipof has received honoraria from Sanofi and has served on the advisory board of AstraZeneca. Dr. Kumar has received research funding from Bristol Myers Squibb and Janssen and has served on the advisory board of AstraZeneca. Dr. Chung has received research funding from Abbvie, Bristol Myers Squibb, Caelum, CarsGen, Cellectis, Janssen, K36 Therapeutics, and Merck Consulting, and he reported consulting activity for Bristol Myers Squibb and Janssen. Dr. Chari has received research funding from Janssen, and he reported consulting activity for Abbvie, Adaptive, Amgen, Antengene, Bristol Myers Squibb, Forus, Genentech/Roche, GSK, Janssen, Karyopharm, Millenium/Takeda, and Sanofi/Genzyme.