Outcomes for relapsed/refractory (R/R) T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) are poor, with limited salvage treatment options and high rates of toxicities.1,2 While immunotherapeutic advances such as blinatumomab,3,4 inotuzumab,5,6 and chimeric antigen receptor T-cell (CAR-T) therapy7 have revolutionized the treatment of newly diagnosed and R/R B-cell ALL, progress in the development of immunotherapies for T-cell malignancies has been hampered by a lack of unique targets, issues with fratricide, inherent toxicities associated with targeting T cells, and the theoretical risk of autologous product contamination with malignant T cells.8 Currently, no immunotherapeutic agents are approved for T-ALL; however, daratumumab, an immunoglobulin G1 kappa human monoclonal antibody targeting CD38 that has been approved by the U.S. Food and Drug Administration for the treatment of multiple myeloma, has shown recent clinical efficacy in T-ALL/T-LBL.9 Recent clinical trials investigating CAR-T therapy for T-cell malignancies have included autologous and allogeneic products targeting commonly expressed T-cell antigens, including CD7, CD5, TRBC1, and CD70.10-18 Among these targets, CD7 is the most frequently expressed in both T-ALL and T-LBL and consistently present at diagnosis, at relapse, and in minimal residual disease (MRD) states.19
WU-CART-007 is an anti-CD7, allogeneic, fratricide-resistant CAR-T product manufactured from healthy donor T cells that are CRISPR-Cas9 edited to delete CD7 and T-cell receptor alpha constant gene, transduced with a second-generation 4-1BB-CD3-zeta CAR targeting CD7, expanded, purified to remove residual T-cell receptor alpha/beta-positive cells, and then cryopreserved. This allows for “off-the-shelf” therapy without the risk of malignant cell contamination and decreases the risks of both fratricide and graft-versus-host disease (GVHD).
This first-in-human, global, multicenter, phase I/II dose escalation and expansion study investigated WU-CART-007 in patients with R/R T-ALL or T-LBL aged 12 years or older. The phase I portion of the study evaluated four dose levels (Table), with the primary objective of characterizing the safety, tolerability, dose-limiting toxicities, and maximum tolerated/maximum administered dose — and determining the recommended phase II dose (RP2D) of WU-CART-007 in T-ALL/T-LBL. The primary objective of the phase II expansion cohort was to evaluate the composite complete remission (CRc) rate in patients with R/R T-ALL/T-LBL.
Best overall response by WU-CART-007 dose (response-evaluable patients)
| Dose Escalation . | Dose Expansion . | . | ||||
|---|---|---|---|---|---|---|
| Cohort . | DL1 100M (n=3) . | DL2 300M (n=3) . | DL3 600M (n=6) . | DL4 900M sLD (n=3) . | RP2D 900M eLD (n=13) . | Total (n=28) . |
| Safety-evaluable * | 3 | 3 | 4 | 3 | 13 | 26 |
| Response-evaluable ** | 3 | 3 | 3 | 3 | 11 | 23 |
| ORR (CR+CRi+PR) | 0 | 2 (66.7%) | 1 (33.3%) | 2 (66.7%) | 10 (90.9%) | 15 (65.2%) |
| CRc (CR+CRi) | 0 | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 8 (72.7%) | 12 (52.2%) |
| CR | 0 | 0 | 1 (33.3%) | 1 (33.3%) | 6 (54.5%) | 8 (34.8%) |
| CRi | 0 | 1 (33.3%) | 0 | 1 (33.3%) | 2 (18.2%) | 4 (17.3%) |
| PR | 0 | 1 (33.3%) | 0 | 0 | 2 (18.2%) | 3 (13.0%) |
| PD | 3 (100%) | 1 (33.3%) | 2 (66.7%) | 1 (33.3%) | 1 (9.1%) | 8 (34.8%) |
| DOR, month, median (95% CI) | - | - | - | - | not reached (0.5, NE) | 6.69 (0.5, NE) |
| Bridged to HCT, n | 0 | 0 | 1 | 1 | 6 | 8 |
| Dose Escalation . | Dose Expansion . | . | ||||
|---|---|---|---|---|---|---|
| Cohort . | DL1 100M (n=3) . | DL2 300M (n=3) . | DL3 600M (n=6) . | DL4 900M sLD (n=3) . | RP2D 900M eLD (n=13) . | Total (n=28) . |
| Safety-evaluable * | 3 | 3 | 4 | 3 | 13 | 26 |
| Response-evaluable ** | 3 | 3 | 3 | 3 | 11 | 23 |
| ORR (CR+CRi+PR) | 0 | 2 (66.7%) | 1 (33.3%) | 2 (66.7%) | 10 (90.9%) | 15 (65.2%) |
| CRc (CR+CRi) | 0 | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 8 (72.7%) | 12 (52.2%) |
| CR | 0 | 0 | 1 (33.3%) | 1 (33.3%) | 6 (54.5%) | 8 (34.8%) |
| CRi | 0 | 1 (33.3%) | 0 | 1 (33.3%) | 2 (18.2%) | 4 (17.3%) |
| PR | 0 | 1 (33.3%) | 0 | 0 | 2 (18.2%) | 3 (13.0%) |
| PD | 3 (100%) | 1 (33.3%) | 2 (66.7%) | 1 (33.3%) | 1 (9.1%) | 8 (34.8%) |
| DOR, month, median (95% CI) | - | - | - | - | not reached (0.5, NE) | 6.69 (0.5, NE) |
| Bridged to HCT, n | 0 | 0 | 1 | 1 | 6 | 8 |
Abbreviations: CR, complete remission; CRc, composite complete remission rate; CRi, complete remission with incomplete hematologic recovery; DL, dose level; DOR, duration of response; eLD, enhanced lymphodepletion; HCT, hematopoietic cell transplantation; M, million; NE, not estimable; ORR, objective response rate; PD, progressive disease; PR, partial response (extramedullary disease only); sLD, standard lymphodepletion.
Two patients, both in DL3, were excluded from analysis as they did not meet World Health Organization criteria for T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma.
Three patients, one in DL3 and two in recommended phase II dose, were not efficacy evaluable as they were discontinued for grade 5 adverse events prior to response evaluation.
A total of 28 patients enrolled and received lymphodepleting chemotherapy followed by a single dose of WU-CART-007. Of the total, 15 patients were treated in the dose escalation cohort and received standard lymphodepleting chemotherapy prior to cell infusion. The remaining 13 patients were treated in the expansion cohort with the RP2D of 900 million cells after receiving enhanced lymphodepleting chemotherapy. The median patient age was 30 years (range, 14-69) and five adolescents were enrolled, all of whom were treated with the RP2D. The study participants had a median of four prior lines of therapy (range, two to seven) and a median bone marrow blast count of 50% (range, 5%-95%). Among all patients, 26.9% had primary induction failure, 38.5% had prior hematopoietic stem cell transplantation (HSCT), 77% had prior nelarabine therapy, and 26.9% had extramedullary disease.
The most common serious adverse events were cytokine release syndrome (CRS; 26.9%) and sepsis (11.5%). Most CRS was grade 1 to 2 (69.2%); grade 3 occurred in three patients (11.5%), with grade 4 occurring in two (7.7%), both of whom fully recovered within seven to 13 days. Median time to CRS onset was 24 hours, and median duration was three days. Increased rates of grade 3 or higher CRS and infection were seen in those patients who received enhanced lymphodepletion. There were two grade 1 immune effector cell-associated neurotoxicity syndrome events (7.7%); one biopsy-confirmed, grade 2 acute skin GVHD (3.8%) in a patient without history of HSCT; and one grade 2 immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome (3.8%), which resolved with anakinra and steroids. Grade 3 and 4 infections occurred in 19.2% of patients, with grade 3 decreased white blood cell count and decreased platelets in 26.9% and 34.6%, respectively. Grade 5 events occurred in three patients (11.5%), including two with invasive fungal infection early in treatment in the setting of persistent disease and one with multi-organ system failure in the setting of rapid disease progression during dose expansion.
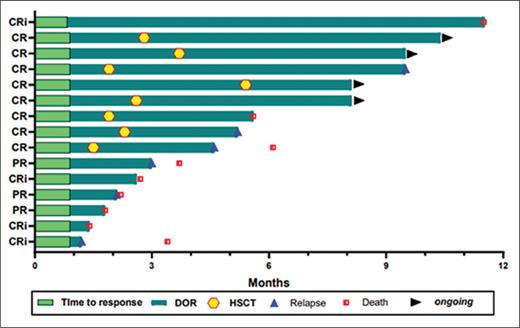
Across all dose levels with 23 response-evaluable patients, the CRc rate was 52.2% and the objective response rate (ORR) was 65.2%. At RP2D with enhanced lymphodepleting chemotherapy, 11 response-evaluable patients had a CRc rate of 72.7% (70% of whom were MRD-negative) and an ORR of 90.9% (Table). The median duration of response was not reached (Figure). Patients with prior HSCT are typically a challenging population to treat, and the ORR among these patients was 88.9% (8/9) and 100% (5/5) for those who received the RP2D. Eight patients in complete remission were bridged successfully to HSCT after WU-CART-007 (three of whom had a prior HSCT) without engraftment delays, resulting in a 100% ORR with count recovery (Figure). One of six patients who relapsed had CD7-negative disease, which is comparable to rates seen in other studies.11,15-18
Swimmer plot of duration of response
Abbreviations: CR, complete remission; CRi, complete remission with incomplete hematologic recovery; DOR, duration of response; HSCT, hematopoietic stem cell transplantation; PR, partial response.
Swimmer plot of duration of response
Abbreviations: CR, complete remission; CRi, complete remission with incomplete hematologic recovery; DOR, duration of response; HSCT, hematopoietic stem cell transplantation; PR, partial response.
In Brief
WU-CART-007, an anti-CD7, allogeneic, fratricide-resistant CAR T-cell product at the RP2D of 900 million cells, was overall well-tolerated and found to have high CRc rates and ORRs (72.7% and 90.9%, respectively) in a heavily pretreated population of patients with R/R T-ALL/T-LBL. Although this study is limited by small numbers, it provides hope for continued advancement in immunotherapeutic options and a cure for this difficult-to-treat population of patients with T-cell malignancies.
Disclosure Statement
The authors indicated no relevant conflicts of interest.