The Case
A 68-year-old woman with chronic obstructive pulmonary disease presented with new-onset night sweats, anorexia, weight loss, and weakness for five months. The patient was found to be anemic, with a hemoglobin level of 7.7 g/dL, normal white blood cell and platelet counts, and 2% circulating blasts. On physical examination, her spleen was painful to palpation and was enlarged 10 cm below the left costal margin. A bone marrow biopsy was hypercellular for age, with marked reticulin fibrosis (grade 2-3 out of 3) and less than 5% bone marrow blasts. The BCR-ABL mutation was not detected, and the karyotype was 46, XX. Next-generation sequencing identified a janus kinase (JAK) 2V617F mutation with a 30% variant allele frequency. These findings confirmed a diagnosis of primary myelofibrosis (MF). The Dynamic International Prognostic Scoring System Plus score was intermediate-2, the Mutation-Enhanced International Prognostic Scoring System Plus version 2.0 score was high risk, and the Genetically Inspired Prognostic Scoring System score was intermediate-1. The patient was not eligible for allogeneic hematopoietic cell transplantation due to impaired lung capacity on pulmonary function testing.
The Question
What is the preferred first-line treatment for symptomatic patients with MF and severe anemia?
The Response
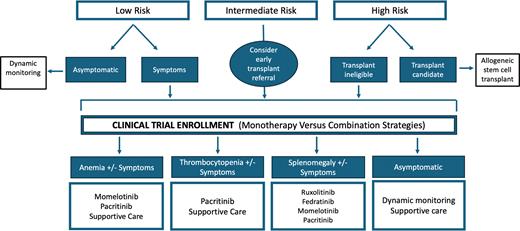
In 2011, the U.S. Food and Drug Administration (FDA) approved ruxolitinib for the treatment of MF. And, for many years, it has been the preferred first-line treatment for symptomatic patients with MF (Figure). The double-blind, randomized COMFORT-I clinical trial of ruxolitinib versus placebo showed significant clinical benefits for patients with intermediate-2 or high-risk MF, with 41.9% of patients in the ruxolitnib group achieving a 35% spleen volume reduction (SVR35) or greater, compared to 0.7% of patients in the placebo group (p<0.001), and 45.9% of those receiving ruxolitnib achieving a 50% reduction in total symptom score (TSS50) or greater at week 24, compared to 5.3% of those receiving placebo (p<0.001).1 In the COMFORT-II randomized clinical trial of ruxolitinib versus best available therapy (BAT), 28% of the patients in the ruxolitinib group had SVR35 or greater at week 48 compared to 0% in the BAT group (p<0.001).2 Although ruxolitinib has shown significant benefits for patients with MF, in the COMFORT-I trial, it was associated with grade 3 and 4 anemia in 45.2% of patients, compared to 19.2% in the placebo group, with approximately half of these hematologic adverse events occurring during the first eight weeks of therapy. Given these data, the recommendation of ruxolitinib for this patient to control her symptoms and spleen size seems reasonable. However, it is also likely to worsen her anemia, leading to dose interruptions, dose reductions, and red blood cell transfusions. Notably, in the COMFORT-I trial, the ruxolitinib dose was adjusted in approximately 70% of patients during the first eight to 12 weeks. Final ruxolitinib doses of 10 mg or greater twice daily were associated with a 31% to 41% median spleen volume reduction at week 24 compared to baseline.3 However, ruxolitinib doses of 5 mg or less twice daily resulted in only a modest median spleen volume reduction (10.4% at week 24). In the REALISE phase II clinical study evaluating novel ruxolitinib dosing strategies, patients with MF, palpable splenomegaly, and hemoglobin levels of less than 10 g/dL were started on a reduced dose (10 mg twice daily for the first 12 weeks) in an effort to mitigate ruxolitinib-associated anemia adverse events. This dosing scheme was followed regardless of the baseline platelet count, allowing for delayed up-titration based on platelet count and efficacy.4 Less than one-fifth of patients required interruption/adjustment for worsening thrombocytopenia (17.6%) and anemia (11.8%), with 56% achieving a 50% or greater reduction in spleen length at week 24.
In 2023, the FDA approved momelotinib for treating patients with intermediate- or high-risk MF with anemia. In addition to inhibiting JAK1 and JAK2, momelotinib selectively inhibits activin A receptor type 1 (ACVR1). Inhibition of ACVR1 reduces hepcidin production, leading to enhanced serum iron availability, which promotes erythropoiesis and alleviates anemia.5,6 The MOMENTUM double-blind, randomized, controlled, phase III clinical trial compared momelotinib to danazol in patients with MF and anemia with a hemoglobin level of less than 10 g/dL.7 Of the 130 patients in the momelotinib arm, 48% were red cell transfusion-dependent. At week 24, 25% of patients in the momelotnib arm achieved TSS50 or greater from baseline, compared to 9% of those in the placebo arm. Furthermore, at week 24, 25% spleen volume reduction was significantly higher in the momelotinib group than in the placebo arm (39% vs. 6%, p<0.0001). Moreover, the SIMPLIFY-1 clinical trial compared momelotinib to ruxolitinib for JAK inhibitor-naive patients with high-, intermediate-2-, or symptomatic intermediate-1-risk MF.8 At week 24, a similar SVR35 was achieved in both arms, demonstrating non-inferiority (26.5% vs. 29%, p=0.011, in the momelotinib vs. ruxolitinib groups, respectively). However, non-inferiority was not met for TSS50 reduction (28.4% vs. 42.2%, p=0.98, in the momelotinib vs. ruxolitinib arms, respectively). Given the coexistence of anemia, offering momelotinib to this patient as a frontline JAK inhibitor is reasonable. When using momelotinib, it is important to monitor treatment-emergent peripheral neuropathy, which was reported in 10% of patients in the momelotinib arm of the SIMPLIFY-1 trial but only 4% of patients in the MOMENTUM trial, with all cases being grade 2 or lower and not leading to drug discontinuation.
If our patient had coexisting thrombocytopenia, we could also consider pacritinib. The PERSIST-2 clinical trial evaluated pacritinib versus BAT, including ruxolitinib, for patients with intermediate-1-, intermediate-2-, and high-risk MF.9 Pacritinib 200 mg twice daily improved both endpoints compared to BAT (SVR35: 22% vs. 3%, p=0.001; TSS50: 32% vs. 14%, p=0.01). It is important to note that ruxolitinib was the most common BAT used in 45% of the study’s patients. Most recently, pacritinib was shown to potently inhibit ACVR1 via downstream SMAD signaling and suppression of hepcidin production.10 In the PERSIST-2 study, patients treated with pacritinib 200 mg twice daily had higher rates of clinical improvement in hemoglobin and reduced transfusion requirements (25% vs. 12% in the pacritinib and BAT arms, respectively).
The Case, Continued
Our patient was started on ruxolitinib according to the REALISE dosing schema. She did not require red blood cell transfusions, with her symptoms resolving shortly after starting treatment. Six months after therapy, the patient’s spleen shrunk to 4 cm below the left costal margin. After three years of ruxolitinib treatment, she started experiencing generalized malaise, fatigue, night sweats, and early satiety. Blood testing showed increased lactate dehydrogenase levels, a stable complete blood count with mild anemia, and 1% peripheral blasts, with physical examination revealing a recurrence of splenomegaly. Repeat bone marrow biopsy and aspiration confirmed the persistence of MF without progression to acute myeloid leukemia.
The Question
What is the preferred second-line treatment for symptomatic patients with MF whose disease has progressed on JAK inhibitor therapy?
The Response
Patients with MF treated with ruxolitinib often develop drug intolerance or disease progression despite a good initial response, with five-year discontinuation rates of up to 75%.11,12 After discontinuation of first-line JAK inhibitors, the median overall survival is less than two years.13 The FREEDOM-2 phase III clinical trial evaluated fedratinib versus BAT in patients with MF previously treated with ruxolitinib.14 At the end of cycle 6, SVR35 was 36% for those receiving fedratinib, compared to 6% for those receiving BAT (p<0.0001); TSS50 reduction was 34% and 17%, respectively (p=0.0033); and SVR35 during the full treatment course was 54% and 12%, respectively (p<0.0001). When using fedratinib, it is essential to monitor and supplement thiamine to prevent thiamine deficiency and to provide antiemetic and antidiarrheal prophylaxis to prevent gastrointestinal side effects. Other JAK inhibitors were also tested for efficacy in second-line treatment following ruxolitinib. Pacritinib in the PERSIST-2 study and momelotinib in the SIMPLIFY-2 and MOMENTUM clinical trials demonstrated SVR35 rates ranging from 7% to 22% and TSS50 rates ranging from 26% to 32%.7,9,15 Enrollment in a clinical trial testing molecules with a novel mechanism of action is encouraged, as survival rates remain low following the failure of first-line JAK inhibitors.
Disclosure Statement
Dr. El Chaer is a consultant for Bristol Myers Squibb, CTI BioPharma, Sobi, and Sumitomo Dainippon. Dr. Rein is a consultant for AbbVie, Cogent Biosciences, Incyte, MorphoSys, Novartis, Sobi, and Sumitomo Dainippon. Dr. Rampal is a consultant for AbbVie, Blueprint, Bristol Myers Squibb, Cogent Biosciences, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma/Sobi, Disc Medicine, Galecto, Incyte, Jazz Pharmaceuticals, Kartos Therapeutics, Karyopharm, Novartis, PharmaEssentia, Promedior, Sierra Oncology/GSK, Stemline Therapeutics, Sumitomo Dainippon, and Zentalis. He received research funding from Biomed Valley Discoveries, Constellation Pharmaceuticals/MorphoSys, Incyte, Ryvu, Stemline Therapeutics, and Zentalis.