STUDY TITLE: DEFIANCE: RCT of ClotTriever System Versus Anticoagulation in Deep Vein Thrombosis (DEFIANCE)
CLINICALTRIALS.GOV IDENTIFIER: NCT05701917
PARTICIPATING CENTERS: 58 centers across the U.S., Finland, Germany, Switzerland, and the U.K.
ACCRUAL GOAL: 300 patients
STUDY DESIGN: DEFIANCE is an industry-sponsored, prospective, randomized controlled trial.1 The trial is recruiting adults with unilateral deep vein thrombosis (DVT) involving the common iliac, external iliac, and/or common femoral vein with symptom onset within three months of enrollment. Included participants will be at least moderately symptomatic, as determined by a Villalta-Prandoni scale (Villalta) score of greater than 9. All participants will undergo computed tomography or magnetic resonance venography to verify criteria for enrollment. Individuals with bilateral iliofemoral DVT, a history of venous stenting, inferior vena cava filter, severe limb compromise, concurrent high-risk pulmonary embolism, congenital anomalies, or a contraindication to therapeutic anticoagulation will be ineligible.
Participants will be randomized 1:1 to undergo either mechanical thrombectomy (MT) of the impacted limb using the ClotTriever® system plus anticoagulation (AC) or initiating/continuing AC alone within 72 hours of randomization. Compression therapy may be used in either arm per the treating provider. Follow-up evaluation will take place at approximately 10, 30, and 180 days from the time of randomization, with remote assessments at 60, 90, 120, and 150 days.
The primary endpoint of the study is a novel, stepwise, composite measure or “win ratio,” incorporating two outcomes in a prespecified hierarchy: 1) treatment failure or therapy escalation and 2) post-thrombotic syndrome (PTS) assessment at six months by Villalta score. Treatment failure is defined by three severe events: venous thromboembolism-related death, amputation of the impacted leg, or development of venous gangrene in the impacted leg. Therapy escalation refers to surgical thrombectomy in either arm or endovascular intervention in the AC arm. To determine the win ratio, pairwise comparisons are conducted between each participant across treatment arms with respect to the treatment failure/escalation. In the event of a “tie” (same outcome in both arms), PTS assessment at six months will be compared. Only “wins” are counted in the final ratio, which divides the number of “winners” in the MT+AC arm by those in the AC arm. The rationale for this complex measure is to ensure that preventing treatment failure/escalation is prioritized over prevention of PTS, while retaining the benefits of a composite measure (the ability to compare multiple endpoints). Reassuringly, the investigators will also report these outcomes as traditional measures to facilitate comparisons with prior trials.
RATIONALE: PTS is a potentially debilitating condition that impacts up to one-half of individuals diagnosed with DVT.2 PTS results from damage to venous valves and vessel walls due to activation of coagulation and inflammation associated with DVT, leading to pooling of blood in the extremities. Symptoms of PTS range from discoloration and varicosities in the impacted limb to heaviness, severe pain, edema, and even chronic ulcers that may lead to missed work and require costly venous interventions and wound care.2 Risk factors for the development of PTS include older age, obesity, preexisting venous insufficiency, extensive proximal (vs. isolated distal) DVT, and inadequate anticoagulation.2
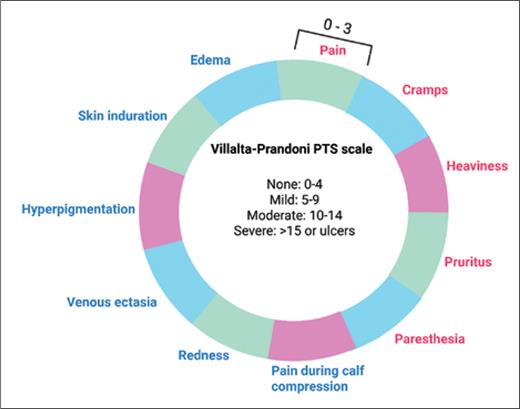
PTS diagnosis is made based on clinical assessment. Due to its ease of use and inter-interpreter reliability, the Villalta scale was adopted by the International Society on Thrombosis and Haemostasis as the preferred method to diagnose PTS and monitor its severity (Figure).2-4 PTS diagnosis is typically deferred until at least six months after an acute DVT due to substantial overlap between symptoms of PTS and acute DVT — and the observation that most acute DVT symptoms resolve with anticoagulation.5
Villalta-Prandoni scale for post-thrombotic syndrome
Abbreviation: PTS, post-thrombotic syndrome. Symptoms (red) and signs (blue) are graded from 0 to 3 and summed for a cumulative score indicating the severity of PTS.
Villalta-Prandoni scale for post-thrombotic syndrome
Abbreviation: PTS, post-thrombotic syndrome. Symptoms (red) and signs (blue) are graded from 0 to 3 and summed for a cumulative score indicating the severity of PTS.
The most effective treatment for PTS is DVT prevention.2 There is limited evidence regarding supervised exercise and conflicting evidence regarding other conservative measures such as elastic compression stockings and horse chestnut derivatives.6-8 Uncertainty regarding optimal management of PTS extends to interventional approaches. ATTRACT, a landmark, multicenter, randomized controlled trial, evaluated pharmacomechanical thrombolysis versus AC alone after acute proximal DVT. No significant difference was found in the development of PTS, as defined by a Villalta score greater than 5 at any point between six and 24 months.9 A follow-up analysis centered on the subgroup of patients with iliofemoral DVT similarly found no benefit with regard to PTS incidence; however, PTS severity scores and venous disease-specific quality of life were significantly improved at 24 months in the thrombolysis arm.10
The use of MT in the management of acute DVT has increased in recent years.1 MT has the potential advantage over thrombolysis in addressing remodeled, chronic thrombus that may be resistant to endogenous and exogenous fibrinolytics.1 Additionally, MT is associated with far less risk of bleeding. The ClotTriever Outcomes (CLOUT) registry reported data on 500 individuals who underwent MT for acute DVT. Comparison between pharmacomechanical thrombolysis and MT was conducted using propensity score matching between individuals enrolled in the ATTRACT trial and those in the CLOUT registry.11 Significant reduction in Villalta scores at 12 months, including 20% more patients without PTS, was observed with MT compared to thrombolysis. The DEFIANCE trial is designed to prospectively evaluate the benefit of MT on PTS.
COMMENT: Standard-of-care DVT management is not effective at preventing PTS in up to a fifth of patients who suffer long-term disability from chronic venous insufficiency.2 Clinical trials of therapies ranging from supervised walking to pharmacomechanical thrombolysis have not yielded much benefit. Observational data on MT is encouraging, with potential benefit for individuals outside of the hyperacute DVT setting.
DEFIANCE aims to provide high-quality evidence evaluating the impact of MT on PTS severity. In a departure from prior studies that examined PTS incidence as the primary outcome, DEFIANCE was designed to detect differences in multiple outcomes prioritizing treatment failure/escalation over PTS severity at follow-up using a win ratio.12 As such, attention will need to be paid to the clinical significance of these findings. In particular, it is notable that subsequent endovascular intervention would count as therapy escalation for individuals in the control arm but not in the thrombectomy arm. The eligibility criteria is designed to enroll individuals at highest risk for PTS, excluding those with femoropopliteal DVT or those with concurrent distal DVT who are at risk for PTS and could benefit from MT. Longer-term follow-up will also be of interest, as PTS symptoms may develop or worsen up to two years following DVT diagnosis.
Disclosure Statement
The authors indicated no relevant conflicts of interest.