The Case
A 20-year-old college student presenting with fever and fatigue was initially treated with antibiotics, but her symptoms persisted. The patient was admitted to the hospital and found to have lymphadenopathy and anasarca. Laboratory results revealed hemoglobin levels of 8 g/dL, a platelet count of 30,000/μL, albumin levels of 2 g/dL, an erythrocyte sedimentation rate of more than 120mm/hr, C-reactive protein (CRP) levels of 153 mg/L, and creatinine levels of 2.1 mg/dL. An antinuclear antibody test, evaluation for lupus, and extensive workup for infectious diseases were all negative. A cervical lymph node biopsy was interpreted as nonspecific, and the patient’s bone marrow showed reactive plasmacytosis. The patient subsequently developed renal and respiratory failure and was transferred to the intensive care unit (ICU), requiring dialysis and ventilation. Empiric treatment with methylprednisolone 2 mg/kg and plasma exchanges were instituted, with response. After one month, the patient was discharged with no definitive diagnosis.
The patient was readmitted six months later with a further crisis. There was a similar clinical picture: high fever, severe fluid overload, and generalized lymphadenopathy. She again required dialysis for renal failure and ventilation. Further complications included septic shock and bowel ischemia. The patient underwent laparotomy, with removal of infarcted small bowel with ileostomy and splenectomy. In addition, she developed a left hemiparesis, with computed tomography showing infarcts in the right corpus callosum, right thalamus, and bilateral gyri. A cytokine panel showed interleukin 6 (IL-6) levels greater than 1,000 pg/mL, which raised the possibility of Castleman disease (CD). A repeat lymph node biopsy was reported as diffuse hyperplasia with prominent plasmacytosis. Expert review suggested plasmacytic-variant CD. Immunostaining for human herpes virus type 8 (HHV-8) was negative. The tumor board reviewed the patient’s clinical and pathology findings and determined that she met the criteria for idiopathic multicentric CD (iMCD). Therapy was instituted with siltuximab and high-dose steroids. The patient recovered, but significant neurological residue remained, as well as short-bowel syndrome. The patient is receiving long-term therapy with siltuximab and is currently doing well.
The Question
How do we improve the diagnosis and outcome of Castleman disease?
The Response
Castleman disease was first described by physician and pathologist Benjamin Castleman, MD, who reported in 1954 on a patient with hyperplastic mediastinal lymph nodes.1 Seventy years later, it has become clear that CD comprises a rare and heterogeneous group of lymph node disorders with shared histopathologic features. Both unicentric and multicentric variants of CD have been described, with a proportion of multicentric cases driven by HHV-8 in the setting of immunosuppression, often due to human immunodeficiency virus.2 In approximately 50% of cases, no cause has been identified thus far; these have been labeled as iMCD. iMCD patients have a systemic inflammatory syndrome and can develop organ dysfunction caused by excess cytokine release. Recently, formal criteria for the diagnosis of iMCD have been published by an international panel of experts convened by the Castleman Disease Collaborative Network (CDCN) (Table).3 These require the presence of lymph node enlargement in two or more lymph node stations, as well as a lymph node biopsy consistent with CD. Additionally, at least two of 11 minor criteria need to be present. These criteria point to inflammatory signs and symptoms, organ dysfunction, hepatosplenomegaly, or skin abnormalities. The histopathology by itself is not diagnostic because similar findings can be observed in malignancies, autoimmune disorders, and infections, all of which require active exclusion. The diagnosis therefore necessitates close collaboration between pathologists and clinicians. Excisional lymph node biopsy is preferred over core biopsies.
Diagnostic criteria for iMCD
| I. Major criteria (need both) |
| 1. Histopathologic lymph-node features consistent with the iMCD spectrum |
| 2. Enlarged lymph nodes (≥1 cm in short-axis diameter) in ≥2 lymph-node stations |
| II. Minor criteria |
| Need at least 2 of 11 criteria, with at least 1 laboratory criterion |
| Laboratory |
| 1. Elevated CRP (>10 mg/L) or ESR (>15 mm/h) |
| 2. Anemia (hemoglobin <12.5 g/dL for males, hemoglobin <11.5 g/dL for females) |
| 3. Thrombocytopenia (platelet count <150 k/μL) or thrombocytes (platelet count >400 k/μL) |
| 4. Hypoalbuminemia (albumin <3.5 g/dL) |
| 5. Renal dysfunction (eGFR <60 mL/min/1.73m2) or proteinuria (total protein 150 mg/24 h or 10 mg/100 ml) |
| 6. Polyclonal hypergammaglobulinemia (total γ globulin or immunoglobulin >1,700 mg/dl) |
| Clinical |
| 7. Constitutional symptoms: night sweats, fever (>38°C), weight loss, or fatigue (≥2 CTCAE lymphoma score for B symptoms) |
| 8. Large spleen and/or liver |
| 9. Fluid accumulation: edema, anasarca, ascites, or pleural effusion |
| 10. Eruptive cherry hemangiomatosis or violaceous papules |
| 11. Lymphocytic interstitial pneumonitis |
| III. Exclusion of diseases that can mimic iMCD |
| 1. Autoimmune disorders (e.g., SLE, rheumatoid arthritis) |
| 2. Infection (e.g., HHV8, CMV, tuberculosis) |
| 3. Malignancy (e.g., Hodgkin and non-Hodgkin lymphoma) |
| I. Major criteria (need both) |
| 1. Histopathologic lymph-node features consistent with the iMCD spectrum |
| 2. Enlarged lymph nodes (≥1 cm in short-axis diameter) in ≥2 lymph-node stations |
| II. Minor criteria |
| Need at least 2 of 11 criteria, with at least 1 laboratory criterion |
| Laboratory |
| 1. Elevated CRP (>10 mg/L) or ESR (>15 mm/h) |
| 2. Anemia (hemoglobin <12.5 g/dL for males, hemoglobin <11.5 g/dL for females) |
| 3. Thrombocytopenia (platelet count <150 k/μL) or thrombocytes (platelet count >400 k/μL) |
| 4. Hypoalbuminemia (albumin <3.5 g/dL) |
| 5. Renal dysfunction (eGFR <60 mL/min/1.73m2) or proteinuria (total protein 150 mg/24 h or 10 mg/100 ml) |
| 6. Polyclonal hypergammaglobulinemia (total γ globulin or immunoglobulin >1,700 mg/dl) |
| Clinical |
| 7. Constitutional symptoms: night sweats, fever (>38°C), weight loss, or fatigue (≥2 CTCAE lymphoma score for B symptoms) |
| 8. Large spleen and/or liver |
| 9. Fluid accumulation: edema, anasarca, ascites, or pleural effusion |
| 10. Eruptive cherry hemangiomatosis or violaceous papules |
| 11. Lymphocytic interstitial pneumonitis |
| III. Exclusion of diseases that can mimic iMCD |
| 1. Autoimmune disorders (e.g., SLE, rheumatoid arthritis) |
| 2. Infection (e.g., HHV8, CMV, tuberculosis) |
| 3. Malignancy (e.g., Hodgkin and non-Hodgkin lymphoma) |
Adapted from Fajgenbaum, et al.3
Abbreviations: CMV, cytomegalovirus; CRP, C-reactive protein; CTCAE, Common Terminology Criteria for Adverse Events; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; HHV-8, human herpes virus type 8; iMCD, idiopathic multicentric Castleman disease; SLE, systemic lupus erythematosus.
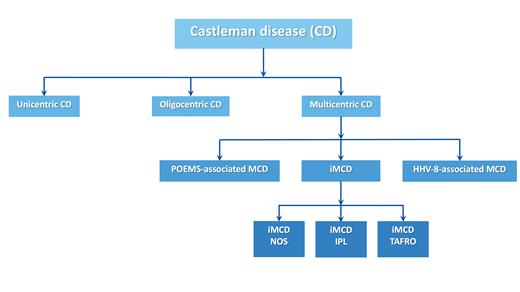
Clinically, iMCD is subdivided into three subtypes (Figure 1). TAFRO syndrome, a severe form of iMCD, is characterized by thrombocytopenia, anasarca, fever or hyperinflammatory state, and organomegaly. Renal dysfunction or reticulin fibrosis of the marrow may also be present.4 Individuals with this condition often have severe flares of cytokine release causing serious organ dysfunction with vascular leak syndrome, leading to hospital admission, ICU stays, and even death. Our patient had several of these features, and the final diagnosis was iMCD-TAFRO. Idiopathic plasmacytic lymphadenopathy (IPL) is a second type of iMCD, in which patients have hypergammaglobulinemia and thrombocytosis, often accompanied by plasma cell infiltration of the lymph nodes.5 The disease course for this type of iMCD is more waxing and waning, or gradually progressive. All other iMCD patients are presently labeled as having iMCD-not otherwise specified. Data from CDCN’s ACCELERATE natural history registry indicate that there is also a more limited form of Castleman disease referred to as oligocentric CD, with patients having only a few lymph node stations involved and no or limited inflammatory symptomatology (Figure 1).6
Updated classification of Castleman disease
Abbreviations: HHV-8, human herpes virus type 8; iMCD, idiopathic multicentric Castleman disease; IPL, idiopathic plasmacytic lymphadenopathy; MCD, multicentric Castleman disease; NOS, not otherwise specified; POEMS, polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes; TAFRO, thrombocytopenia, anasarca, fever, reticulin fibrosis or renal insufficiency, and organomegaly.
Updated classification of Castleman disease
Abbreviations: HHV-8, human herpes virus type 8; iMCD, idiopathic multicentric Castleman disease; IPL, idiopathic plasmacytic lymphadenopathy; MCD, multicentric Castleman disease; NOS, not otherwise specified; POEMS, polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes; TAFRO, thrombocytopenia, anasarca, fever, reticulin fibrosis or renal insufficiency, and organomegaly.
Historically, one-third of iMCD patients have succumbed to the disease within five years.7 To date, a multitude of therapies have been used to treat iMCD, including corticosteroids, rituximab, chemotherapy, immunomodulatory drugs, and monoclonal antibodies (mAbs) such as rituximab. IL-6 and other cytokines are responsible for the inflammatory syndrome and associated constitutional symptoms, and mAbs have been used to target the IL-6 signaling cascade. Tocilizumab is a humanized antibody that targets the IL-6 receptor and has been approved for the treatment of iMCD in Japan based on a single-arm study of 28 patients.8 Additionally, siltuximab, a chimeric antibody that directly neutralizes IL-6, has been studied in a global phase II randomized, double-blind, placebo-controlled study. Durable tumor and symptomatic responses occurred in 18 (34%) of 53 siltuximab-treated patients, compared to zero of 26 in the placebo arm.9 A recent post-hoc analysis showed superior two-year progression-free survival in patients receiving siltuximab (91% vs. 37%; p=0.0001).10 A long-term extension study of 60 patients enrolled in phase I or II studies showed that 42 (70%) continued to have long-term responses, with a follow-up of six years.11 Patients coming off of the medication for any reason were considered a treatment failure, including eight individuals who transitioned to commercial siltuximab. Only two patients experienced disease progression while on siltuximab. Overall, the drug was well-tolerated, with no cumulative toxicity. Patients most likely to respond to siltuximab have evidence of an inflammatory response, e.g., elevated CRP, low hemoglobin, low albumin, or elevated fibrinogen.12
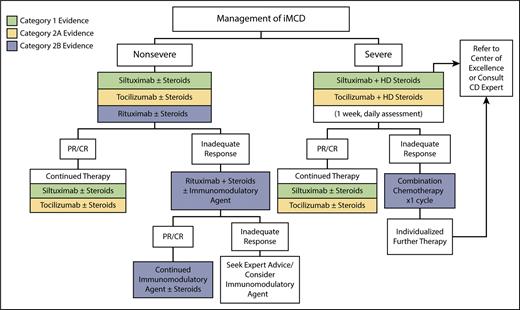
A lack of clarity regarding the most appropriate therapy for iMCD prompted physicians from the CDCN to formulate international evidence-based treatment guidelines, taking into account published literature, reported clinical trials, and expert opinion. Corticosteroid monotherapy had the lowest response rate. Chemotherapy and anti-IL-6 antibody therapy had similar response rates (63% and 61%, respectively), but the treatment failure rate associated with chemotherapy was much higher (42% vs. 32%).13 Based on these considerations, the treatment algorithm recommends first-line treatment with an anti-IL-6 mAb (Figure 2). Preference is given to siltuximab over tocilizumab, as it is the most comprehensively studied agent in iMCD. Recommended second-line therapy in patients with non-severe iMCD comprises rituximab and steroids, with or without an immunomodulatory agent. Patients with severe disease often have iMCD-TAFRO syndrome. Consideration should be given to an accelerated dosing schedule with an anti-IL-6 mAb and high-dose steroids.14 CRP levels should be measured to follow response, rather than IL-6 levels, as siltuximab spuriously elevates IL-6.15 Failure of therapy may necessitate the administration of combination chemotherapy.
Treatment algorithm for idiopathic multicentric Castleman disease
Originally featured in van Rhee, et al.13
Abbreviations: HD, high-dose; PR/CR, partial response/complete response.
Treatment algorithm for idiopathic multicentric Castleman disease
Originally featured in van Rhee, et al.13
Abbreviations: HD, high-dose; PR/CR, partial response/complete response.
Conclusion
The key to diagnosing rare disorders is physician awareness, which helps ensure prompt recognition. Recently formulated guidelines for both the diagnosis and treatment of iMCD should lead to improved future outcomes.
Disclosure Statement
Dr. van Rhee has received consultancy fees and research funding from Recordati/EUSA.