STUDY TITLE: A Study of Ianalumab (VAY736) in Patients With Primary Immune Thrombocytopenia (ITP) Previously Treated With at Least Two Lines of Therapies
CLINICALTRIALS.GOV IDENTIFIER: NCT05885555
PARTICIPATING CENTERS: International study with 24 participating centers
SPONSOR: Novartis Pharmaceuticals
ACCRUAL GOAL: A total of 41 participants have been enrolled
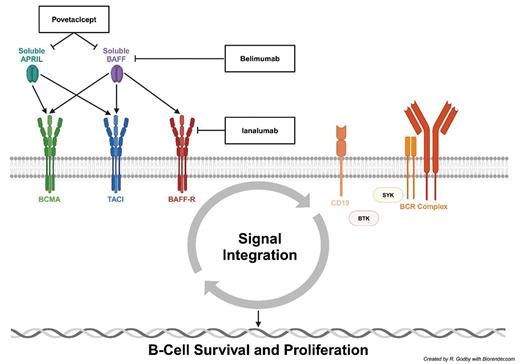
STUDY DESIGN: Ianalumab, a novel monoclonal antibody targeting the B-cell activating factor (BAFF, or B lymphocyte stimulator [BlyS]) and its receptor (BAFF-R), is currently being investigated for the treatment of primary immune thrombocytopenia (ITP) and other immune dysregulation disorders (Figure 1).
Ianalumab mechanism of action and current autoreactive B-cell targets for the treatment of immune thrombocytopenia
Abbreviations: APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; BAFF-R, B-cell activating factor receptor; BCMA, B-cell maturation antigen; BCR, B-cell receptor; BTK, Bruton tyrosine kinase; SYK, spleen tyrosine kinase; TACI, transmembrane activator and calcium-modulator and cyclophilin ligand (CAML) interactor.
Ianalumab mechanism of action and current autoreactive B-cell targets for the treatment of immune thrombocytopenia
Abbreviations: APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; BAFF-R, B-cell activating factor receptor; BCMA, B-cell maturation antigen; BCR, B-cell receptor; BTK, Bruton tyrosine kinase; SYK, spleen tyrosine kinase; TACI, transmembrane activator and calcium-modulator and cyclophilin ligand (CAML) interactor.
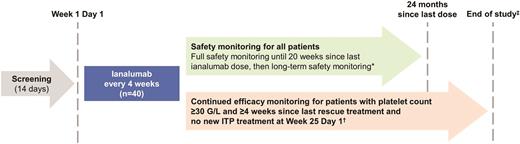
VAYHIT3 is a phase II, open-label, single-arm, multicenter clinical trial designed to evaluate the efficacy, safety, and tolerability of intravenous ianalumab in adults with primary ITP previously treated with at least a corticosteroid and thrombopoietin receptor agonist (TPO-RA) who have a spleen and require additional therapy (Figure 2).1 The primary outcome measure is a confirmed response (platelet count of 50g/L or higher on at least two consecutive assessments seven days apart). The secondary outcome measures include time to confirmed response, duration of confirmed response, response rates, bleeding events, the need for rescue treatment, changes in CD19+ B cells and immunoglobulins, and anti-ianalumab antibodies.
VAYHIT3 trial design1
*Long-term safety monitoring includes only the collection of adverse events (AEs) and serious adverse events (SAEs) potentially related to B-cell depletion or assessed by the investigator as related to auxiliary medicinal products (AMPs) and SAEs assessed by the investigator as possibly related to ianalumab. If a different B cell-depleting therapy starts, AEs and SAEs assessed by the investigator as related to AMPs and SAEs assessed by the investigator as possibly related to ianalumab will be collected.
†Efficacy monitoring will end if, after Week 25 Day 1, the patient’s platelet count is less than 30 g/L, the patient starts a new line of immune thrombocytopenia therapy (ITC), or the patient requires a rescue treatment.
‡The study will end once all patients have completed 24 months of safety follow-up since their last dose of ianalumab or have been discontinued from the study earlier.
VAYHIT3 trial design1
*Long-term safety monitoring includes only the collection of adverse events (AEs) and serious adverse events (SAEs) potentially related to B-cell depletion or assessed by the investigator as related to auxiliary medicinal products (AMPs) and SAEs assessed by the investigator as possibly related to ianalumab. If a different B cell-depleting therapy starts, AEs and SAEs assessed by the investigator as related to AMPs and SAEs assessed by the investigator as possibly related to ianalumab will be collected.
†Efficacy monitoring will end if, after Week 25 Day 1, the patient’s platelet count is less than 30 g/L, the patient starts a new line of immune thrombocytopenia therapy (ITC), or the patient requires a rescue treatment.
‡The study will end once all patients have completed 24 months of safety follow-up since their last dose of ianalumab or have been discontinued from the study earlier.
RATIONALE: Primary ITP is an acquired platelet disorder characterized by immune-mediated destruction of platelets leading to thrombocytopenia without an alternative etiology. Clinical sequelae, such as mucocutaneous bleeding, typically manifest when platelet counts are less than 20 g/L.
The pathophysiology underlying ITP is multifaceted, stemming from immune dysregulation that leads to both impaired megakaryopoiesis and increased peripheral destruction of platelets. While the inciting triggers for primary ITP remain elusive, antiplatelet antibodies produced by autoreactive B cells – augmented by autoreactive T cells – play a critical role.
Treatment for ITP aims to prevent bleeding by achieving platelet levels sufficient for hemostasis. Currently, initial treatment strategies primarily rely on glucocorticoids. While efficacious, responses are not universal, with only a fraction of patients achieving long-term durability.2 Subsequent management strategies include therapies that stimulate megakaryopoiesis (e.g., TPO-RAs) or abrogate immune-mediated platelet destruction by restoring immune homeostasis, often via the targeting of autoreactive B cells (e.g., rituximab). If neither approach is adequately effective, a splenectomy may be considered.
However, the ITP armamentarium continues to expand, with several therapies targeting autoreactive B cells in various treatment settings. Fostamatinib inhibits spleen tyrosine kinase (SYK) (Figure 1), which is important for both Fc gamma receptor signaling in macrophages and B-cell receptor (BCR) signaling, and is already approved by the U.S. Food and Drug Administration (FDA) for the treatment of chronic ITP requiring additional therapy. Rilzabrutinib inhibits Bruton tyrosine kinase (BTK) (Figure 1), which is important for both BCR and CD19 signaling, and is currently under investigation for the treatment of ITP. Povetacicept is a monoclonal antibody against both soluble BAFF and a proliferation-inducing ligand (APRIL) (Figure 1), both of which are elevated in ITP, and is also currently under investigation for ITP.3
Similarly, belimumab is a monoclonal antibody against soluble BAFF that is already approved by the FDA for the treatment of systemic lupus erythematosus (SLE) and results in decreased signaling through BAFF-R, B-cell maturation antigen (BCMA), and transmembrane activator and calcium-modulator and cyclophilin ligand (CAML) interactor (TACI) (Figure 1).
Ianalumab is an immunoglobulin G1 monoclonal antibody that directly inhibits the BAFF-R (Figure 1). The duality of its mechanism of action is unique in that it both depletes B cells via antibody-dependent cellular cytotoxicity to a higher degree than rituximab and inhibits signaling through BAFF-R.4 It has the potential to induce deep and durable remissions in primary ITP and is also being studied in the frontline setting (NCT05653349).
Additionally, ianalumab is being studied for the treatment of warm autoimmune hemolytic anemia (NCT05648968), chronic lymphocytic leukemia (NCT03400176), and autoimmune diseases such as Sjogren’s syndrome (NCT02962895) and SLE (NCT03656562).
COMMENT: Great progress has been made in the treatment of primary ITP, and there is likely more to come in addition to the targeting of autoreactive B cells, including targeting plasma cells (anti-CD38 molecules), pathogenic antibodies (anti-neonatal Fc receptor molecules), and the complement system. However, more therapies are needed, and sequencing as well as combinatorial strategies require exploration for optimal use in this burgeoning field. There is currently an unmet need for safe therapies capable of reproducibly eliciting deep, durable responses for patients with immune dysregulation disorders. Collaborations both within and outside of hematology will continue fostering progress at the collective intersection of interventional immunology.
Disclosure Statement
Drs. Godby and Shah indicated no relevant conflicts of interest.