The Cases
Case 1
A 25-year-old man with hemoglobin SS (HbSS), a severe form of sickle cell disease (SCD), presents for his annual hematology visit with a complaint of morning headaches and poor sleep for the past six months. He denies vision changes, chest pain, dyspnea, focal weakness, and lower extremity edema. He has had recurrent acute chest syndrome (ACS) episodes, stuttering priapism as a teenager, and unprovoked pulmonary embolism (PE). He takes hydroxyurea 1,500 mg daily (maximum tolerated dose), folic acid, apixaban, and acetaminophen and oxycodone as needed for pain. His most recent labs showed a hemoglobin of 8.3g/dL, absolute reticulocyte count (ARC) of 275 x109/L, white blood cell count of 6 x109/L, and a lactate dehydrogenase level (LDH) of 495 U/L. At the visit, baseline oxygen saturation (SpO2) and six-minute walk distance were within normal limits. He ultimately completed nocturnal polysomnography that revealed nocturnal hypoxemia (SpO2 <89%) for 10 minutes of sleep time (mean SpO2 of 90%) without obstructive sleep apnea (apnea-hypopnea index <5).
Case 2
A 34-year-old woman with SCD (HbSS with hereditary persistence of fetal hemoglobin) reports an upcoming long-distance flight for vacation. Her disease is complicated by avascular necrosis of the right shoulder and proliferative sickle retinopathy. She has had a history of hypersplenism, managed medically. She recalls two prior long-distance flights, one of which was complicated by a vaso-occlusive event (VOE) 48 hours after arrival at her destination. She has no known pulmonary hypertension, obstructive sleep apnea, asthma, or renal disease and she is a non-smoker. Her body mass index is 24. She has an average of two VOEs per year. Her most recent labs are notable for hemoglobin of 10 g/dL, ARC of 150x109/L, white blood count of 8x109/L, and LDH level of 350 U/L. Prior echocardiogram, pulmonary function tests (PFTs), and overnight polysomnography were unremarkable. She underwent high-altitude simulation testing (HAST) that uncovered hypoxemia (SpO2 <89%).
The Questions
Case 1
When should we suspect and screen for hypoxemia in people living with SCD?
Case 2
What do we do about the risk of altitude-associated hypoxemia in people with SCD?
The Responses
Case 1
Hypoxemia, or low blood oxygen levels, can arise from cardiac or pulmonary complications in SCD.1 As people with SCD are living longer, cardiopulmonary complications have been identified as significant predictors of mortality.2-4 Therefore, it is important to have a high index of suspicion for these complications, including hypoxemia.
Hypoxemia is a feature of acute complications of SCD, such as ACS, PE, and pneumonia. However, there are chronic complications (often occult) that can also be characterized by low oxygen levels, including pulmonary hypertension, obstructive and restrictive lung disease, and sleep-disordered breathing (SDB).1 Nocturnal and post-exertional hypoxemia has been reported in children and young adults with SCD,5-10 even in the absence of acute and chronic comorbidities. The presence of hypoxemia in the absence of “classical” SDB (i.e., elevated apnea-hypopnea index)8,9 has been associated with cerebrovascular, cardiovascular, and genitourinary complications in children.5,11-14 In those studies, individuals with hypoxemia typically had more severe SCD genotypes (HbSS and HbS beta-zero thalassemia) and phenotypes (hemolysis, reticulocytosis, priapism, and central nervous system events).
Current evidence- and expert-based guidelines15,16 do not recommend routine screening for cardiopulmonary complications in asymptomatic children and adults. However, clinical experience suggests that history-taking and symptom interrogation are often incomplete; thus, subtle symptoms and signs may be overlooked during routine clinical visits.
In patients with symptoms or signs concerning for hypoxemia or other respiratory complaints (Figure 1), our own evolving clinical practice is to assess with targeted history and physical exam; this guides the choice of diagnostic testing, the need for subspeciality referral, and eventual management. While not relevant to this case, the index of suspicion for hypoxemia should remain high in pregnant people with SCD as well. Pregnancy carries an increased risk for maternal and fetal morbidity and mortality in people with SCD.17 The pathophysiological changes of pregnancy can amplify the underlying disease activity. Management guidelines17-19 recommend baseline assessment of oxygen saturation and close monitoring of oxygen levels in the intrapartum period.
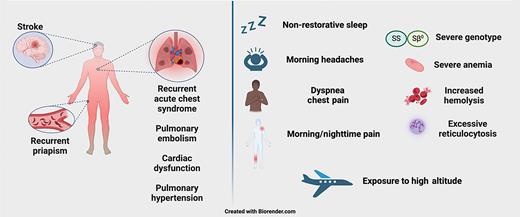
When to consider hypoxemia testing? Clinical factors that increase suspicion for occult hypoxemia in people with sickle cell disease
When to consider hypoxemia testing? Clinical factors that increase suspicion for occult hypoxemia in people with sickle cell disease
Symptoms concerning for hypoxemia include morning headaches, nighttime/morning pain, and nonrestorative sleep. Concerning respiratory symptoms include dyspnea, chest pain, exercise limitation, and syncope/presyncope. Clinical history concerning for hypoxemia includes history of recurrent ACS, history of PE, history of ischemic stroke, and recurrent priapism. Lab findings that may coincide with hypoxemia include severe anemia (hemoglobin <8g/dL), excessive reticulocytosis (ARC >180 ×109/L),20 or increased hemolysis (elevated LDH).
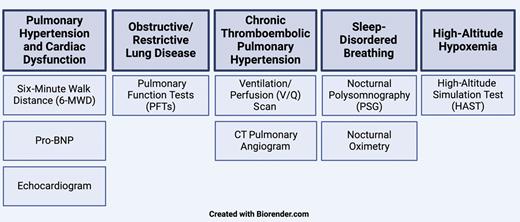
Diagnostic tests to consider include the following (Figure 2; routine tests are bolded):
Pulmonary hypertension and cardiac/valvular dysfunction: six-minute walk distance (6-MWD), pro-brain natriuretic peptide (proBNP), echocardiogram, right-heart catheterization when indicated
Obstructive/restrictive lung disease: comprehensive PFTs, computed tomography (CT) of the chest when indicated
Chronic thromboembolic pulmonary hypertension: ventilation/perfusion scan, CT pulmonary angiogram
SDB: nocturnal polysomnography, nocturnal oximetry
Hypoxemia: nocturnal oximetry, polysomnography, 6-MWD, HAST
In this case, the following are suggestive of risk for hypoxemia: morning headaches, history of recurrent ACS, history of priapism and PE, and labs indicating excessive reticulocytosis and hemolysis. Further evaluation to consider in this case included 6-MWD, nocturnal oximetry and/or polysomnography, and CT of the chest, if indicated. The discovery of nocturnal hypoxemia without SDB was managed with nocturnal oxygen supplementation, with gradual improvement in the patient’s symptoms.
Case 2
High altitude — during airplane flights or mountainous destinations — is associated with reduced oxygen levels or “hypobaric hypoxia.” This can exacerbate sickling of red blood cells and potentially increase the risk of VOEs and other SCD-associated complications. Published case reports21,22 and patient anecdotes have described VOEs and splenic symptoms after exposure to high altitudes, suggesting an association with antecedent hypoxemia. The prevalence of high-altitude hypoxemia is unknown, and there is a need for comprehensive studies to guide systematic recommendations to manage high-altitude exposure in patients with SCD.
Our pre-travel assessment in people with SCD includes obtaining a detailed history of prior travel and associated complications, as well as of other cardiopulmonary conditions23 such as pulmonary hypertension, chronic obstructive pulmonary disease, and heart failure. We routinely screen with HAST, which can uncover hypoxemia at simulated exposure to 8,000 feet for 20 minutes (most airplanes are pressurized to 5,000 feet above sea level).24 Our preliminary work25 identified hypoxemia during HAST in more than 55% of 26 individuals tested over three years. In the absence of evidence-based guidelines, HAST results can guide the shared decision-making process, including the need for supplemental oxygen therapy while in flight or at altitude.
In this case, the patient’s history of VOEs after a prior flight increased the potential risk for hypoxemia and SCD complications with exposure to high altitude. Given the development of hypoxemia (SpO2 <89%) during the test, she was prescribed supplemental oxygen for use during the flight. In addition, we discussed access to health care facilities and resources at the travel destination, in case of complications.
Conclusion
Hypoxemia may insidiously, and deleteriously, affect patients living with SCD; thus, it is prudent for providers to maintain awareness of this complication. Our current clinical practice combines a detailed history and physical exam with targeted but thorough cardiopulmonary testing. In our shared SCD-pulmonary medicine clinic, we provide interventions (with nocturnal, exertional, or travel oxygen) and multidisciplinary care to support our patients. Overall, these interventions appear to have subjectively improved the health and symptom burden of our patients. However, these approaches await rigorous and prospective evaluation, which we and others are pursuing.
Competing Interests
Drs. Obadina, Wilson, LeVarge, and Little indicated no relevant conflicts of interest.