A 23-year-old man presented with leukopenia and thrombocytopenia persisting for four years. He complained of occasional epistaxis and easy bruising every few months. He had no history of medication. An extensive prior workup at an outside hospital, including HIV testing, bone marrow biopsy, flow cytometry for paroxysmal nocturnal hemoglobinuria, and assessments for rheumatologic and infectious diseases, yielded negative results. Abdominal ultrasound demonstrated hepatomegaly (17.7 cm) and splenomegaly (15.2 cm). Serum vitamin B12 was elevated (2,841 pg/mL; reference range: 232-1,245 pg/mL). A complete blood count with differential revealed normal red blood cell count and hemoglobin (5.27 × 109/L and 16.7 g/dL, respectively), leukopenia (2.5 × 109/L; neutrophils: 1.03 × 109/L; lymphocytes: 1.14 × 109/L) and thrombocytopenia (89 × 109/L). Bone marrow aspiration with biopsy was performed (Figure). Cytogenetic study showed normal karyotype. A next-generation sequencing of a myeloid panel revealed no somatic mutations.
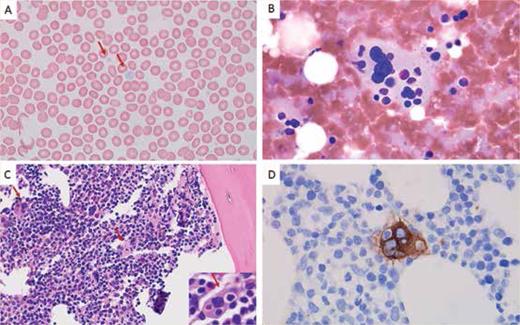
Peripheral blood showed agranular platelets with variable size (A, Wright stain, 1000×). Bone marrow touch preparation showed a megakaryocyte with prominent (B, Giemsa stain, 1000×). Bone marrow biopsy showed normocellular bone marrow (70%) with trilineage hematopoiesis and frequent emperipolesis in megakaryocytes (C, hematoxylin and eosin stain, 400×; inset, 1000×). The CD61 stain highlights megakaryocyte emperipolesis (D, immunohistochemistry with hematoxylin counterstain, 1000×).
Peripheral blood showed agranular platelets with variable size (A, Wright stain, 1000×). Bone marrow touch preparation showed a megakaryocyte with prominent (B, Giemsa stain, 1000×). Bone marrow biopsy showed normocellular bone marrow (70%) with trilineage hematopoiesis and frequent emperipolesis in megakaryocytes (C, hematoxylin and eosin stain, 400×; inset, 1000×). The CD61 stain highlights megakaryocyte emperipolesis (D, immunohistochemistry with hematoxylin counterstain, 1000×).
What is the diagnosis?
May-Hegglin anomaly
GATA1-related cytopenia
Gray platelet syndrome
Bernard-Soulier syndrome
Answer: Gray platelet syndrome (GPS) is a rare autosomal recessive disorder caused by homozygous or compound heterozygous mutation in the NBEAL2 gene on chromosome 3p21.1 GPS is characterized clinically by mild to moderate bleeding symptoms, macrothrombocytopenia, splenomegaly, elevated serum vitamin B12 levels and bone marrow fibrosis. The median age of presentation in a large study cohort was 11.5 years, but the age varied between 2 months and 67 years.2 Elevated vitamin B12 levels have been noted in 66% of patients, often exceeding 1.5 times the upper limit of the local reference range.2 Splenomegaly and bone marrow fibrosis each affect approximately half of patients with GPS. Peripheral blood smears reveal platelets of enlarged size with a characteristic gray appearance due to decreased alpha-granule content. Emperipolesis, the safe passage of intact cells through the cytoplasm of another cell, is frequently observed in megakaryocytes.3 Additional clinical phenotypes associated with GPS include reduced leukocyte counts and increased presence of autoimmune disease and positive autoantibodies.2
Our patient exhibited several hallmark features of GPS, including: agranular, large platelets in peripheral blood; hepatosplenomegaly; elevated serum vitamin B12; frequent emperipolesis in megakaryocytes; and evidence of marrow fibrosis (MF-1). Given these findings, germline testing for NBEAL2 mutations is strongly advised to confirm the diagnosis and guide appropriate management strategies.
Choice A (May-Hegglin anomaly) is incorrect.
Choice B (GATA1-related cytopenia) is incorrect.
Choice D (Bernard-Soulier syndrome) is incorrect.
Competing Interests
Drs. Wei and Ok indicated no relevant conflicts of interest.
For the solution to the quiz, visit The Hematologist online at www.hematology.org/TheHematologist/Image-Challenge.