STUDY TITLE: A Phase I/II Study of Trametinib and Azacitidine for Patients with Newly Diagnosed Juvenile Myelomonocytic Leukemia (T2020-004)
CLINICALTRIALS.GOV IDENTIFIER: NCT05849662
PARTICIPATING CENTERS: Therapeutic Advances in Childhood Leukemia Consortium sites in the United States
SPONSOR: Therapeutic Advances in Childhood Leukemia (TACL) Consortium
ACCRUAL GOAL: 64 participants
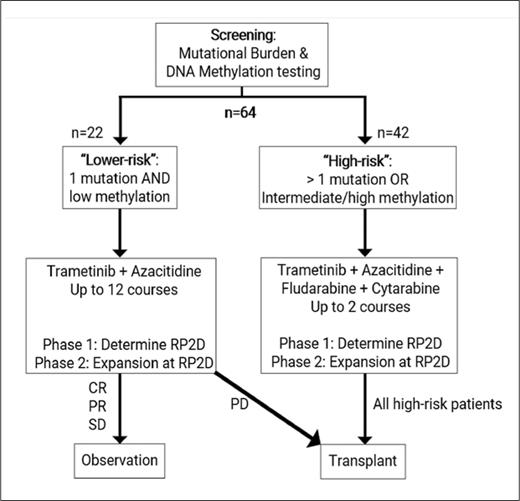
STUDY DESIGN: T2020-004 is a risk-stratified, non-randomized phase I/II clinical trial designed to examine the safety and efficacy of the MEK1/2 inhibitor trametinib in combination with other agents for children and young adults (age >1 month and <21 years) with newly diagnosed juvenile myelomonocytic leukemia (JMML). Patients are risk stratified according to mutational burden and DNA methylation. Mutational burden is defined based on the number of somatically mutated genes and the number of clonal alterations on cytogenetic testing. DNA methylation is defined in accordance with international consensus criteria.1 Lower-risk cases are defined as those involving one somatic alteration and “low” DNA methylation. High-risk cases are defined as those involving either more than one somatic alteration or “intermediate/high” DNA methylation. The lower-risk and high-risk arms of the study are independent phase I/II studies with their own respective objectives.
Patients with lower-risk JMML will be treated with azacitidine for five days in combination with trametinib for 28 days per course for up to 12 courses. These patients will only proceed to hematopoietic stem cell transplant (HCT) in the setting of progressive disease. The primary objective of the lower-risk arm is to determine the safety of combining trametinib with azacitidine. The secondary objective is to describe event-free survival in patients treated with trametinib and azacitidine who do not proceed to HCT within one year.
Patients with high-risk JMML will be treated with azacitidine for five days followed by fludarabine and cytarabine for five days in combination with daily trametinib for 28 days per course, proceeding to HCT after two courses of therapy. The primary objective of the high-risk arm is to determine the safety of combining trametinib with azacitidine, fludarabine, and cytarabine. The secondary objective is to determine the rate of molecular response prior to HCT.
Clinical trial schema for T2020-004 investigating the combination of azacitidine and trametinib in children and young adults with newly diagnosed JMML
Clinical trial schema for T2020-004 investigating the combination of azacitidine and trametinib in children and young adults with newly diagnosed JMML
Regimen for Lower-risk Cases
| Days (Courses 1-12) . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | … . | 28 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine | • | • | • | • | • | |||||||||
| Trametinib | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Days (Courses 1-12) . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | … . | 28 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine | • | • | • | • | • | |||||||||
| Trametinib | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
Regimen for High-risk Cases
| Days (Courses 1 & 2) . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | … . | 28 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine | • | • | • | • | • | |||||||||
| Trametinib | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Fludarabine | • | • | • | • | • | |||||||||
| Cytarabine | • | • | • | • | • |
| Days (Courses 1 & 2) . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | … . | 28 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine | • | • | • | • | • | |||||||||
| Trametinib | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Fludarabine | • | • | • | • | • | |||||||||
| Cytarabine | • | • | • | • | • |
RATIONALE: JMML is an aggressive myeloproliferative neoplasm of childhood characterized by aberrant signaling through the Ras pathway caused by germline and somatic driving mutations in NF1, NRAS, KRAS, RRAS, RRAS2, SH2B3, PTPN11, and CBL. Despite upfront treatment with HCT, event-free survival for patients with newly diagnosed JMML has historically hovered around 50% at three years post-HCT, with the most common event being relapse. However, in rare cases, patients who have undergone observation without HCT have experienced long-term survival (termed spontaneous resolution). Recent studies have shown that mutational burden2-4 and DNA methylation5,6 are remarkably predictive of these different outcomes. Patients who experience spontaneous resolution of their disease always have low DNA methylation. Patients with more than one somatic alteration or intermediate/high methylation have particularly poor outcomes, with relapse occurring in approximately two-thirds to three-fourths of cases.
A trial conducted in Europe recently demonstrated excellent outcomes in pediatric patients with JMML who received azacitidine as pre-HCT therapy.7 A trial conducted in the United States also recently demonstrated the efficacy of trametinib monotherapy in pediatric patients with relapsed and refractory JMML.8 Preclinical studies have shown potential synergy between these two agents.9 Based on these results, the current trial combines azacitidine with trametinib alone in lower-risk cases and in combination with chemotherapy followed by HCT in high-risk cases.
COMMENT: Despite the observation that certain patients with JMML can achieve long-term survival in the absence of HCT, all clinical trials conducted worldwide to date have mandated transplant for every patient with this disease. International collaborative efforts have identified mutational burden and DNA methylation as important predictors of outcome. This trial represents the first risk-stratified treatment strategy for patients with JMML and is the first to use this novel, biologically relevant combination of azacitidine and trametinib. Patients with lower-risk disease will have the option to avoid HCT in the absence of progressive disease. Patients with high-risk disease, which has historically been associated with dismal outcomes, will receive these targeted agents in combination with chemotherapy (fludarabine and cytarabine) in an effort to increase the number of patients achieving molecular remission prior to HCT, which has been associated with improved overall outcomes.
Competing Interests
Drs. Stieglitz and Pommert indicated no relevant conflicts of interest.