Anti-CD20 monoclonal antibodies have been a cornerstone in the bag of tricks against B-cell lymphoma for 25 years2 and have laid the foundation for other therapeutic strategies targeting B-cell-restricted antigens. These include CD19-targeting therapies such as chimeric antigen receptor T cells (CART19)3 and, most recently, CD20xCD3 T-cell-engaging bispecific antibodies.
Mosunetuzumab, a bispecific antibody that directs endogenous CD3+ T cells to malignant CD20+ B cells, recently gained accelerated approval for relapsed and refractory (R/R) follicular lymphoma.4 However, B-cell lymphomas often have other tricks up their sleeves when treated with CD20-and CD19-targeting immunotherapies,5,6 including target escape. In their recent study, Stephen J. Schuster, MD, and colleagues demonstrated that mosunetuzumab is also vulnerable to this mechanism of treatment resistance.1
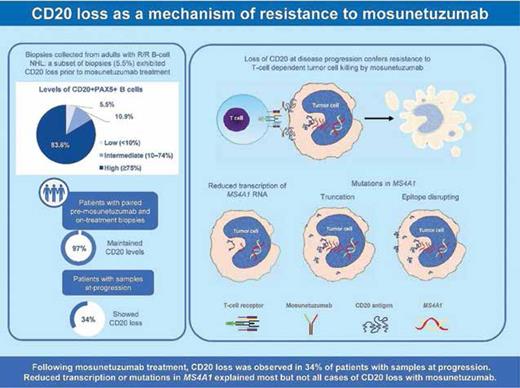
In the G029781 trial,4 CD20 loss based on immunohistochemistry (IHC, <10%) was uncommon (5.5%) in pretreatment biopsies from patients with R/R B-cell lymphomas but was associated with refractory disease. IHC-confirmed CD20 loss was identified in 34% (11/32) of biopsies at progression, occurring more frequently in patients with aggressive lymphomas. Progression-free survival was also shorter for patients with CD20 loss.
To look behind the curtain, the authors performed whole-exome sequencing (WES) and RNA sequencing (RNAseq) on tumor biopsies. A total of 10 patients harbored CD20 variants before treatment, though none had CD20 loss. In contrast, four of five patients with CD20 variants after treatment experienced CD20 loss. RNAseq of MS4A1 encoding CD20 demonstrated that gene expression was correlated with IHC-confirmed levels of CD20. Most cases of discordance (CD20 loss with RNA expression) were explained by underlying mutations. Reductions in gene expression were observed in 21.4% (6/28) of progression biopsies, mostly in those from non-responders. Most instances of CD20 variants or transcriptional downregulation led to CD20 loss at progression, but there were also cases of CD20 loss without these underlying causes.
To functionally characterize this game of smoke and mirrors, the authors introduced four mutations into a diffuse large B-cell lymphoma (DLBCL) cell line, located at or near the extracellular loops. The two point mutations blocked extracellular anti-CD20 binding, while the frameshift and truncating mutations both abrogated CD20 protein expression. The point mutations reduced levels of CD20xCD3-induced T-cell activation and cell killing, which were instead absent with the non-expressing genetic variants.
Whether CD20 variants were acquired or their presence at subcloncal levels was enriched due to selective pressure remains unknown.5 All patients had previously received an anti-CD20 antibody, though not necessarily with their latest treatment. The study by Dr. Schuster and colleagues relied on WES, meaning that potential variants in non-coding regions were not identified. Epigenetic changes (e.g., promoter methylation) can also lead to reduced expression, in turn resulting in CD20 loss without mutation. While the authors defined CD20 loss according to IHC results, flow cytometry (FCM) is another clinical methodology that can be used to detect CD20. Even when IHC results for CD20 are positive, FCM results may be negative in some cases of DLBCL, and this discordant immunophenotype is associated with decreased gene expression of MS4A1 and sensitivity to rituximab.7 CD20 loss was only observed in a minority of cases of progression after mosunetuzumab; however, T-cell exhaustion and mutations in other genes affecting the tumor-immune microenvironment are also possible mechanisms of relapse.8
In Brief
In this study, Dr. Schuster and colleagues demonstrated that CD20 loss was uncommon before mosunetuzumab treatment for R/R B-cell lymphomas but emerged in a third of patients at the time of progression. Transcriptional downregulation or mutations in the encoding gene MS4A1 explained some but not all instances of CD20 loss (Figure). Functional characterization studies indicated that point mutations in the extracellular ECL2 loop, which contains the binding epitope for mosunetuzumab, can lead to treatment resistance without CD20 loss.
Visual Abstract. Incidence of CD20 loss before and during mosunetuzumab therapy and at progression. CD20-specific mechanisms of treatment resistance are also depicted (reprinted with permission from Schuster SJ, et al. Blood. Published online December 4, 2023).
Visual Abstract. Incidence of CD20 loss before and during mosunetuzumab therapy and at progression. CD20-specific mechanisms of treatment resistance are also depicted (reprinted with permission from Schuster SJ, et al. Blood. Published online December 4, 2023).
As seen after other immunotherapies, B-cell lymphomas can also make CD20 disappear when exposed to mosunetuzumab (and likely other CD20xCD3 bispecific antibodies).9,10 In clinical practice, reviewing CD19 and CD20 status at the time of progression is crucial for selecting the appropriate T-cell redirecting agents, and combining IHC and FCM may improve sensitivity for antigen loss. Deep, targeted sequencing of tissue or circulating tumor DNA may also help to identify variants that predict treatment resistance.5 In an elegant recent study, Johannes Duell, MD, and colleagues showed that sequential antigen loss can occur in patients receiving CART19 and CD20xCD3 bispecific antibodies, with re-emergence of CD20 representing branching evolution from selective pressures.11 Moving forward, focusing on multiple antigens simultaneously may aid in preventing tumor targets from vanishing in a puff of smoke.12,13 Continued study of the mechanisms underlying treatment resistance is needed to keep the magic alive as novel therapeutic agents are developed for B-cell lymphomas.
Competing Interests
Dr. Herrera has received consulting fees from Genentech-Roche. Dr. Cherng indicated no relevant conflicts of interest.