Recurrent gastrointestinal bleeding is a frequent cause of iron deficiency anemia, with small-intestinal bleeding accounting for 5 to 10% of all patients presenting with gastrointestinal bleeding, which is especially frequent in older adults.1,2 Patients with small-intestinal angiodysplasia (SIA) and other gastrointestinal vascular malformations frequently present with obscure gastrointestinal bleeding. Recent advancements in imaging and endoscopy, including video capsule and angiography techniques, have led to improved identification of the source. However, treatment of these lesions continues to pose a significant clinical challenge given that local interventions such as ablation, embolization, and surgical resection tend to carry a risk of recurrent bleeding episodes without amelioration of the issue, perhaps due to the multicentric nature of the disease.
SIA is characterized by the accumulation of abnormally dilated blood vessels in the submucosa and mucosa of the small intestine. Although the exact mechanisms are unknown, SIA is thought to develop as a result of increased contractility within the muscularis propria, leading to chronic obstruction of the veins within the submucosa and subsequent congestion at the level of the capillaries, thus leading to development of arteriovenous collaterals via angiogenesis. Due to high levels of vascular endothelial growth factor (VEGF) in individuals with SIA, antiangiogenic therapies, including somatostatin analogues, have been investigated as a potential treatment modality. Thalidomide, which also has antiangiogenic properties, is thought to suppress the production of both VEGF and the transcription factor NF-κB, thereby leading to the induction of apoptosis.3
In recent years, several case reports described the use of thalidomide for preventing recurrent gastrointestinal bleeding, citing encouraging clinical outcomes. These studies prompted a single-center, open-label, parallel controlled trial out of Shanghai, China, in which patients with gastrointestinal vascular malformations (including angiodysplasia and/or gastric antral vascular ectasia [GAVE]) with recurrent/refractory bleeding were randomized to receive thalidomide or iron supplementation (control cohort).4 Among the 52 patients with SIA, the effective response rate — defined as the proportion of patients in whom there was a reduction of at least 50% in bleeding events within the first year following treatment when compared with the number of bleeding events in the observation year pre-treatment — was observed in 69.2% of patients in the thalidomide cohort, versus only 3.8% in the control cohort. Moreover, 11 patients (42.3%) receiving thalidomide experienced a cessation of bleeding, compared with 0 in the control arm.
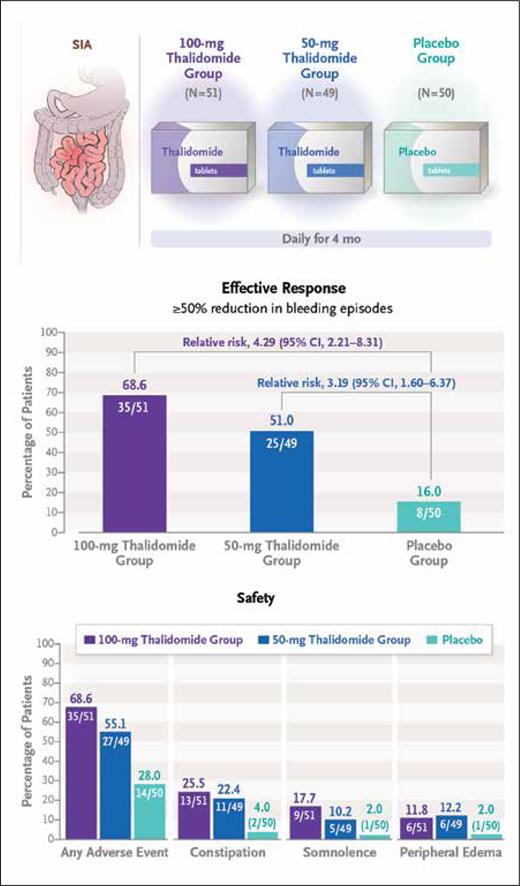
These findings ultimately prompted the multicenter, double-blind, randomized control trial by Huimin Chen, MD, and colleagues, who investigated the long-term efficacy and safety of thalidomide as a therapeutic strategy for recurrent bleeding caused by SIA. The investigators randomized 150 patients who had experienced at least four bleeding events secondary to SIA over the preceding year (i.e., the observation year) to receive either 50 mg or 100 mg of daily oral thalidomide for months and compared outcomes relative to placebo treatment.5 Clinical outcomes were assessed for one year following end of treatment. The primary endpoint was effective response, defined as an at least 50% reduction in bleeding events during the one-year post-treatment period (vs. the observation period). Significant differences in the primary endpoint were observed, with rates of 68.6% and 51.0% in patients who received the 100-mg and 50-mg doses of thalidomide, respectively, compared with a rate of 16.0% in the placebo arm. An analysis of secondary endpoints revealed a need for blood transfusions in 17.6% and 24.5% of patients in the 100-mg and 50-mg thalidomide groups, respectively, compared with a rate of 62.0% in the placebo arm. Similarly, rates of hospitalization secondary to bleeding during the one-year follow-up period were 27.5% and 34.7% in the 100-mg and 50-mg thalidomide groups, respectively, compared with a rate of 74.0% in the placebo group. While there were no serious adverse events, grade 1 and 2 adverse events such as limb numbness, somnolence, peripheral edema, and constipation were frequently noted in the thalidomide cohort, although they quickly resolved following the end of the treatment period.
While this study demonstrated evidence supporting the use of thalidomide for recurrent bleeding secondary to SIA, there were several limitations to the trial, such as the inclusion of bleeding events defined solely based on positive fecal occult blood tests, which may be clinically insignificant. However, occult bleeding accounted for less than 20% of bleeding events, and subsequent analyses excluding occult bleeding events produced results similar to those observed in the primary analysis. Additional limitations included a small sample size, restriction to a single ethnicity (Han Chinese), and the exclusion of individuals with certain predisposing conditions to the development of SIA, including aortic stenosis and hereditary hemorrhagic telangiectasia.
Summary of trial schema and efficacy and safety outcomes (reprinted with permission from Chen H, et al. N Engl J Med. Published online November 2, 2023).
Summary of trial schema and efficacy and safety outcomes (reprinted with permission from Chen H, et al. N Engl J Med. Published online November 2, 2023).
In Brief
Preventing recurrent small-intestinal bleeding from angiodysplasias using local therapies remains a therapeutic challenge. While systemic therapy with somatostatin analogues has demonstrated modest efficacy in reducing transfusion needs in this patient population, additional effective and safe alternatives are needed.6 Thalidomide may confer a therapeutic effect by suppressing increases in VEGF expression in patients with angiodysplasias, thereby inhibiting angiogenesis. In this study by Dr. Chen and colleagues, patients with SIA who had experienced recurrent bleeding events were randomized to receive either 50 or 100 mg of oral thalidomide or placebo. Significant reductions in bleeding events and transfusion needs were observed among those receiving thalidomide, suggesting its safety and efficacy against persistent or recurrent bleeding caused by SIA. However, additional trials are required to determine the duration of therapy necessary to achieve a clinical benefit, the maximal dose of thalidomide, and whether retreatment is necessary.
Competing Interests
Drs. Harris and Shah indicated no relevant conflicts of interest.