Next-generation sequencing technologies for studying the genome and transcriptome have greatly enhanced our understanding of biological processes and are already being applied in clinical practice. Similar efforts targeting proteins, especially post-translational modifications (PTMs), are increasing in both clinical and research settings. This is especially important because protein abundance does not always correlate with transcript levels due to a multitude of post-transcriptional and post-translational mechanisms. Moreover, PTMs are not encoded in the genome. Although this necessitates the development of specific strategies for measuring them, investigating PTMs can provide deeper insights into the signaling mechanisms that drive pathological processes.
Mass spectrometry has become the preferred and gold standard analytical tool for proteomic analysis. Significant developments over the last several years have led to the emergence of newer technologies that enable highly sensitive, reproducible, and in-depth proteomic analysis of various biological processes. Here, we describe some recent developments in proteomic technologies that have the potential to improve our investigations of molecular systems and their impact on hematology research and clinical practice.
High-throughput and Large-scale Proteomic Analysis
Although proteomic methods have largely been used to analyze small sets of samples ranging from cells and tissues (including formalin-fixed paraffin-embedded [FFPE] blocks) to body fluids, recent efforts have focused on increasing the throughput with a simultaneous increase in the depth of coverage of proteins and PTMs. Recent developments in mass spectrometry acquisition methods, such as the inclusion of a data-independent acquisition (DIA) mode, have enabled reproducible detection and quantitation of approximately 8,000 proteins from limited numbers of cells (1,000 cells). This methodology is generally applied in “label-free” experiments, in which the samples are analyzed via individual mass spectrometry runs, following which the protein intensity signals are integrated across the samples. Label-free experiments are more appropriate when the number of samples is large, allowing the DIA approach to improve the quantitative performance of proteomic analysis with fewer missing values across samples when compared with traditional data-dependent acquisition (DDA) methods.
However, because the complete human proteome has millions of peptides, any additional separation can allow for deeper coverage. Ion mobility is one such technology, which adds another dimension of peptide separation within the mass spectrometer by exploiting the differential mobility of ions under the influence of nitrogen gas and electric field. Ion mobility technology, especially parallel accumulation serial fragmentation (PASEF), coupled to DIA analysis is termed diaPASEF workflow. This innovative approach has enabled significantly higher proteome coverage within individual samples, resulting in the identification of up to 10,000 proteins in a single run.1 Integrative diaPASEF workflows also allow for deeper analysis of biofluids like plasma, cerebrospinal fluid (CSF), and urine, which have traditionally been less explored due to the wide, dynamic range of protein abundance.2
In contrast to label-free experiments, multiplexed approaches are routinely used for global proteomic analysis when the number of samples is limited. In tandem mass tag (TMT)-based strategies, peptides from each sample are barcoded with a unique reporter tag, following which all labeled samples are pooled and subjected to tandem mass spectrometry analysis. Protein quantitation across the samples is deduced from the reporter ion signals of the identified peptides. Currently, TMT-based strategies are limited to 18 samples per experimental set, meaning that analyses of large cohorts require multiple experimental sets. Indeed, TMT-based strategies have been extensively applied in discovery studies of various cancer types and have been further integrated with genome and transcriptome analyses to gain significant insights into the relevant tumorigenic mechanisms.3
As technological advancements in mass spectrometry continue to influence proteomic studies, emphasis on the development of innovative, automated, and high-throughput sample processing workflows has increased, with an additional goal of enhancing reproducibility. Our group has recently developed an automated, high-throughput sample processing workflow using adaptive-focused acoustics technology for low-input laser capture-microdissected samples.4 This technique enables processing of up to 96 samples simultaneously and significantly decreases the overall sample processing time to under 4 hours (vs. 24 hours in routine workflows). In addition, liquid handling systems that support the automation of sample processing workflows (e.g., AssayMap Bravo, KingFisher, and Microlab STAR Liquid Handling System) are rapidly being developed. To date, proteomic analysis has not been commonly applied to hematologic cancers, especially in comparison with transcriptome analysis, mostly owing to the limited amounts of samples that are generally available. The technologies discussed above hold significant promise in facilitating the comprehensive profiling of the entire proteome across hundreds of samples, and the integration of proteomic, genomic, and transcriptomic data may bring us closer to obtaining a holistic understanding of these diseases.
Exploration of the PTM Landscape
PTMs are covalent protein modifications that play several important roles via alterations in protein structure, function, and localization. Examples of important PTMs include phosphorylation, glycosylation, ubiquitylation, acetylation, and methylation. Kinases drive the phosphorylation of proteins important in regulating key molecular mechanisms and represent an important class of druggable therapeutic targets, especially in the context of individualizing therapies.5 Similarly, ubiquitylation is critical in regulating protein levels, thus controlling key biological processes in the cell. Studies of ubiquitylation have expanded our understanding of the pleiotropic effects of the immunomodulatory drugs (IMiDs) that are commonly used in multiple myeloma treatment.6 Mass spectrometry-based profiling of the ubiquitylome in multiple myeloma cell lines treated with lenalidomide has revealed altered ubiquitylation of IKZF1 and IKZF3 by CRL4CRBN E3 ubiquitin ligase.6 Similarly, glycosylation of monoclonal light chains has been demonstrated to be a predictor of susceptibility to light chain amyloidosis and plasma cell disorders.7
Given their importance, in-depth analysis of these PTMs is challenging and often requires larger sample amounts for enrichment of modified peptides due to their presence at low stoichiometric levels. Immobilized metal ion or metal oxide affinity chromatography is commonly used for phosphopeptide enrichment, enabling the detection of more than 20,000 phosphosites from as little as 10 μg of protein (100,000 cells). Immunoaffinity approaches using antibodies specific to PTM residues are also commonly used to enrich modified peptides. For example, enrichment of ubiquitylated peptides followed by TMT-based multiplexing can enable identification of approximately 20,000 ubiquitylation sites from 500 μg of input sample.8 Similarly, antibodies specific to phosphotyrosine and acetylated lysine residues are routinely used for global analyses of PTMs. Using automation systems such as the KingFisher platform, these methods can now be scaled to process up to 96 samples in a single run.
Single-cell Proteomics
Proteomic analysis has hitherto centered primarily around bulk samples, resulting in a loss of essential information related to the cellular heterogeneity present within the tissue. While several single-cell proteomic platforms such as mass cytometry, cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq), RNA expression and protein sequencing assay (REAP-seq), and IsoPlexis are available for targeted quantitation of multiple proteins, mass spectrometry is the analytical tool currently used for unbiased global proteomics at the single-cell level. Since the average protein content per cell is typically in the picogram range, minimizing any potential losses and optimizing protein recovery during sample preparation are critical for single-cell proteomic approaches. Despite the availability of single-cell isolation methods such as robotic cell isolation, laser capture microdissection, fluorescence-based cell sorting, and microfluidic devices, the lack of a reliable sample processing workflow has greatly limited single-cell proteomic analysis.
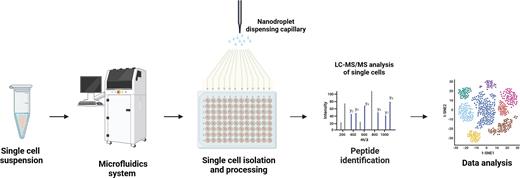
To address this issue, novel workflows that miniaturize sample processing to the nanodroplet level have been developed, thereby enhancing protein recovery.9 One commercially available platform, cellenONE, combines picoliter dispensing with image-based sorting of individual cells and sample processing. This integrated workflow ultimately generates peptides suitable for mass spectrometry analysis (Figure 1). In addition, the newest-generation mass spectrometers provide greatly enhanced sensitivity for single-cell proteomic analysis.10, 11 Early studies on single-cell proteomics have already reported quantifying approximately 3,000 proteins per cell. Thus, there is great promise that further technological advancements can enhance both the throughput and depth of single-cell proteomic analysis and make routine work of studying PTMs at the single-cell level. Overall, integration of single-cell proteomics with other single-cell omics modalities has the potential to revolutionize biomedical research,12 especially in hematology — from the study of cellular lineages and heterogeneity to mechanisms of drug resistance and tumor development.
Single-cell proteomics. A schematic representing the workflow for single-cell proteomic analysis. After isolation of single cells, proteins from each cell are digested into peptides and subjected to mass spectrometric analysis.
Single-cell proteomics. A schematic representing the workflow for single-cell proteomic analysis. After isolation of single cells, proteins from each cell are digested into peptides and subjected to mass spectrometric analysis.
Imaging Mass Spectrometry for Studying Spatial Biology
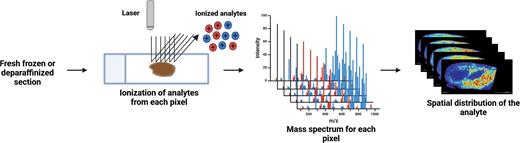
Imaging mass spectrometry is a powerful tool for investigating the spatial distribution of proteins, peptides, lipids, metabolites, glycans, and drugs in a biological specimen. Matrix-associated laser desorption ionization (MALDI) coupled to time-of-flight (TOF) mass spectrometry is the most popular platform for imaging analysis. The typical experimental workflow employed in imaging experiments is depicted in Figure 2. Protein imaging has unique challenges due to the complex nature of the proteins; however, a MALDI Hi-plex immunohistochemistry (IHC) workflow has recently been developed to study the spatial distribution of proteins.13 As in traditional immunohistochemistry, this workflow involves staining the section with antibodies; however, the antibodies are crosslinked to photocleavable mass tags, which are then cleaved after staining, and the slides are analyzed using MALDI-based mass spectrometry. This workflow can be scaled to study the spatial distribution of up to 200 protein markers in a single experiment.
MALDI imaging analysis of a tissue section. In a typical imaging workflow, tissue sections (fresh-frozen or deparaffinized) are sprayed with a chemical matrix to aid analyte desorption and ionization. A specific area of the section (referred to as a pixel) is then irradiated by a laser beam in the MALDI source, recording a mass spectrum of ionized analytes. This process is repeated in a rasterizing pattern across the tissue section, and analyte abundances across all pixels are overlaid to generate a spatial image of the analyte distribution.
MALDI imaging analysis of a tissue section. In a typical imaging workflow, tissue sections (fresh-frozen or deparaffinized) are sprayed with a chemical matrix to aid analyte desorption and ionization. A specific area of the section (referred to as a pixel) is then irradiated by a laser beam in the MALDI source, recording a mass spectrum of ionized analytes. This process is repeated in a rasterizing pattern across the tissue section, and analyte abundances across all pixels are overlaid to generate a spatial image of the analyte distribution.
One of the technological challenges in imaging mass spectrometry is achieving high spatial resolution, which is crucial for obtaining detailed molecular information at the cellular and subcellular levels. While a spatial resolution of 20 μm is common for such imaging analyses, a key technological advancement has enabled a spatial resolution of 5 μm, pushing the limits closer to single-cell resolution. This technology can also be used to study sequential sections of a sample, facilitating a three-dimensional view of the analyte distribution within the sample. In addition, following MALDI imaging, the tissue section can be stained with hematoxylin and eosin to integrate histological features with molecular measurements. Overall, imaging mass spectrometry is a valuable tool for understanding the spatial organization of molecules within a tissue section that can be further integrated with genomic, transcriptomic, and proteomic techniques to provide a comprehensive understanding of complex biological systems.
Impact of Proteomic Analysis on Hematology Research and Clinical Practice
In the context of clinical practice, proteomic analysis offers the potential for stratifying patients and monitoring disease prognosis as well as treatment responses. Identification of protein biomarkers can also aid in early detection and risk assessment, potentially enabling timely intervention. Mass spectrometry has already been recognized as a clinically validated assay for the diagnosis of multiple myeloma, based on measurements of the monoclonal protein (M-protein) produced by abnormal plasma cells, and has been suggested as an alternative to immunofixation electrophoresis by the International Myeloma Working Group.14 In addition, mass spectrometry methods can be used to detect amyloid proteins in FFPE biopsy specimens from patients with amyloidosis.15
Conclusion
While genomic and transcriptomic analyses have received considerable attention and have been widely employed to examine disease mechanisms involved in hematologic disorders, proteomic analyses have yet to be applied on a similar scale. Nonetheless, proteomic technologies can enable comprehensive protein profiling, thereby shedding light on disease mechanisms and aiding in the identification of novel biomarkers and potential therapeutic targets. In recent years, the use of spatial biology approaches has also increased, allowing us to gain critical insights into the complex interactions between tumor cells and the microenvironment. Overall, the integration of proteomic analysis with other omics data holds significant promise for advancing our understanding and management of hematologic disorders.
Competing Interests
Drs. Mangalaparthi and Pandey indicated no relevant conflicts of interest.