Allogeneic hematopoietic stem cell transplantation (HCT) has been established as a curative therapy in patients with hematologic malignancies and marrow failure syndromes. Ideally, HCT patients are cured of their underlying condition, remain free of graft-versus-host disease (GVHD), and have normal hematopoietic and immune function, thus affording them a high quality of life after treatment. Despite advances in the field, these ideal outcomes are not attainable for every patient, and novel strategies for preventing and treating GVHD remain a key research focus.
On behalf of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN), Dr. Javier Bolaños-Meade and colleagues conducted a multicenter, randomized, phase III study involving 431 adult patients undergoing reduced-intensity allogeneic HCT, predominantly for acute leukemia and myelodysplastic syndromes.1 Participants with a median age of 66 years were randomized to receive either standard GVHD prophylaxis2 (i.e., treatment with the calcineurin inhibitor tacrolimus and methotrexate) or experimental prophylaxis (i.e., post-transplant cyclophosphamide [PT-Cy] followed by tacrolimus and mycophenolate mofetil [MMF]).
Pharmacological prophylaxis against GVHD aims to limit donor T-cell responses to recipient antigens — a biological process critical to the pathogenesis of GVHD. Administration of PT-Cy on days 3 and 4 post-transplant has been pioneered in the haploidentical setting, in which only half of human leukocyte antigen (HLA) alleles are matched between the recipient and a related donor (such as a parent or child).3 Mechanistically, PT-Cy depletes and suppresses the function of highly proliferative alloreactive donor cells and is also thought to spare the emerging regulatory T-cell compartment.4 Years of use in the haploidentical setting have demonstrated that PT-Cy-based approaches are safe and effective for the prevention of GVHD. The study by Dr. Bolaños-Meade and colleagues defines the role of PT-Cy-based GVHD prophylaxis in patients undergoing reduced-intensity conditioning outside the haploidentical setting. The overwhelming majority of patients received matched grafts: 66.8% from unrelated donors and 29.7% from family members. Just 3.5% received mismatched grafts from unrelated donors (i.e., only 7/8 identical HLA alleles).
In Brief
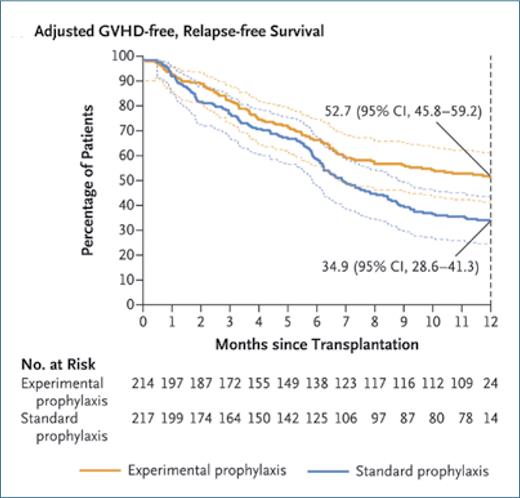
The authors report that the cohort treated with PT-Cy/tacrolimus/MMF had significantly lower rates of severe acute GVHD (grades 3 and 4), chronic GVHD, relapse, and death from any cause than the cohort treated with tacrolimus/methotrexate (Figure). The composite primary endpoint of GVHD- and relapse-free survival at 1 year was met in 52.7% of patients in the PT-Cy/tacrolimus/MMF cohort and 34.9% of patients in the tacrolimus/methotrexate cohort. The work of Dr. Bolaños-Meade and colleagues builds on prior findings demonstrating the promise of this approach, offering strong evidence that the PT-Cy/tacrolimus/MMF strategy yields superior outcomes in this patient population.
Competing Interests
Dr. Markey indicated no relevant conflicts of interest.