STUDY TITLE: A Phase III, Multicenter, Randomized, Double-Blind, Placebo Controlled, Parallel-Group Study with an Open-Label Extension to Evaluate the Efficacy and Safety of Oral Rilzabrutinib (PRN1008) in Adults and Adolescents with Persistent or Chronic Immune Thrombocytopenia (ITP) (LUNA3)
CLINICAL TRIALS.GOV IDENTIFIER: NCT04562766
PARTICIPATING CENTERS: 129 study locations in 25 countries
ACCRUAL GOAL: 224 participants
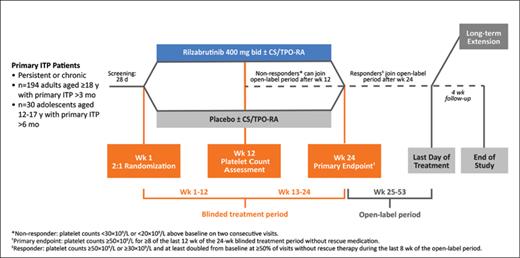
STUDY DESIGN: This ongoing phase III, placebo-controlled, double-blind, randomized controlled trial aims to compare the efficacy of rilzabrutinib (treatment arm) vs. placebo (control arm) for the treatment of primary, persistent immune thrombocytopenia (ITP). Briefly, eligibility criteria include an age of 10 years or older; diagnosis of primary, persistent ITP; non-sustained response to firstline therapy (corticosteroids or intravenous immunoglobulin [IVIg]) and documented intolerance, insufficient response, or contraindication to other appropriate standard therapies for ITP; an average platelet count less than 30 × 103/mm3; adequate hepatic and renal function; absolute neutrophil count (ANC) greater than 1.5 × 109/L; and hemoglobin (Hb) level greater than 9 g/L. Secondary ITP, pregnancy/lactation, solid organ transplant, myelodysplastic syndromes, and previous or active malignancy likely to require treatment during the study are considered exclusion criteria. Patients on stable doses of corticosteroid therapy or thrombopoietin receptor agonists (TPO-RAs) will also be considered eligible. However, those taking immunosuppressant drugs other than corticosteroids and those who have received treatment with rituximab or undergone a splenectomy within three months prior to Study Day 1 will be considered ineligible. Finally, patients must not have received treatment with an alternate Bruton tyrosine kinase (BTK) inhibitor within 30 days prior to Study Day 1.
Patients will be randomized 2:1 to receive twice-daily oral treatment with 400 mg of either rilzabrutinib or placebo for up to 24 weeks, followed by a 28-week open-label period. Outside the E.U. and U.K., the primary outcome measure is a durable platelet count response in the last six weeks of the 24-week blinded treatment period. For LUNA3, durable platelet response is defined as the proportion of participants able to achieve platelet counts at or above 50 × 103/mm3 for over two-thirds of at least eight measurements, without rescue therapy. Within the E.U. and U.K., the primary outcome measure is the proportion of patients achieving platelet counts at or above 50 × 103/mm3 for at least eight of the last 12 weeks of the 24-week blinded treatment period. Numerous secondary outcome measures have been outlined, including time-to-response, the proportion of patients requiring rescue therapy, patient-reported quality-of-life (based on the ITP-Patient Assessment Questionnaire in adult patients and ITP-KIT score in pediatric patients), bleeding events, and treatment-related adverse events. Patients exhibiting a response to treatment by the end of the open-label period are eligible for inclusion in a long-term extension study and will continue to receive treatment until the last patient entering the long-term extension study has completed 12 months of follow-up. The Figure summarizes the study design.1
LUNA3 Phase III Study Design
Kuter DJ et al. Blood. 2021;138(Supplement 1):1010.
RATIONALE: ITP is characterized by autoimmune-mediated destruction of platelets and impaired platelet production, putting patients at risk of bleeding, decreased quality-of-life, and death.2 Firstline treatment options for ITP (i.e., corticosteroids and IVIg) are rarely associated with durable responses.3 While the number of available treatments for ITP continues to increase, treatment responses remain variable and infrequently sustained, and many of these agents are associated with notable toxicities.3,4 Hence, there is still an urgent need for novel ITP treatments that are both safe and effective.
Since the discovery of ibrutinib in 2007, BTK inhibitors have transformed the treatment landscape for a number of B-cell hematologic malignancies.5 Improved understanding of the molecular biology of BTK and the role of BTK regulation in autoimmunity have sparked an interest in BTK-inhibition as a therapeutic target in patients with autoimmune diseases.6 Rilzabrutinib is an oral, reversible BTK inhibitor specially designed for the treatment of autoimmune diseases.7 Importantly, the high specificity and rapid systemic clearance of this agent decrease the risk of off-target toxicities, and it does not alter platelet aggregation.4,7 Last year, The New England Journal of Medicine published the results of a phase I/II trial of rilzabrutinib for the treatment of ITP, reporting that 40% of treated patients exhibited a platelet-count response (i.e., at least two consecutive platelet counts >50 × 103/mm3 and an increase of >20 × 103/mm3 from baseline without the use of rescue medication).4 Given these findings and the low-level toxicities observed, the authors concluded that BTK inhibition may play a role in the treatment of ITP and that rilzabrutinib appears to be safe and effective. LUNA3 is the follow-up phase III, double-blind, randomized controlled trial comparing rilzabrutinib with placebo.
COMMENT: This ambitious trial aims to establish rilzabrutinib as an alternative treatment option for patients with persistent ITP who have not had durable responses to firstline therapies. Although the treatments were not compared head to head, the response rate of 40% observed in the phase I/II trial was lower than that of established second-line treatments for ITP,2 suggesting that rilzabrutinib is unlikely to be a complete “game-changer.” However, the safety and tolerability data are very encouraging, and the potential for durable responses is enticing,8 indicating that rilzabrutinib may represent a useful addition to the therapeutic armamentarium for ITP. The investigators should be commended for the inclusion of validated patient-reported outcome measures among their secondary outcomes. Among the trial’s potential limitations is the use of different primary outcome measures depending on the location of the study center (inside vs. outside the U.K./E.U.), though this was done for regulatory reasons and results will be handled together for analysis of drug efficacy (obviating an impact on the statistical power of the study).
Competing Interests
Dr. Scott has received consulting fees from Amgen.