Foreword From Editor-in-Chief Shaji Kumar, MD
Hematologic disorders encompass myriad conditions ranging from the mundane to the exotic, often broadly grouped into benign or malignant — though the distinction has increasingly become blurred with the recognition of premalignant conditions that straddle the divide between benign and malignant blood disorders.
For a long time, the malignant conditions have enjoyed the limelight for a variety of reasons, not the least being the fear associated with the “C” word and the perceived mortality associated with many of the disorders. At the same time, the rapid strides that have been made in understanding the disease mechanisms and increasing treatment options for malignant disorders have kept them in the spotlight, attracting further interest and focus. However, what is often not perceived is the tremendous impact of non-malignant blood disorders on patients due to a combination of the overall burden, the chronic symptomatology associated with the disease as well as its treatment, and the economic burden resulting from these chronic conditions.
While these disorders are often thought of as “benign,” many of these conditions are anything but. For example, many of the hemoglobinopathies, while not fatal, significantly impact quality of life, present tremendous economic burden to society, cause much suffering for those affected, and most importantly reduce the life span of the affected individuals. Disorders of coagulation can lead to significant morbidity through their clinical manifestations and their sequelae, whether they are excessive bleeding or clotting. With the debate over what to call these disorders (i.e., classical, nonmalignant, or benign), as highlighted by the letter to the editor from Dr. Steven Lentz and the response from Dr. Robert Brodsky, we want to introduce a new series titled “Not So Benign” to draw the attention of the hematology community to these less appreciated disorders and increase awareness of the biology as well as treatment approaches. We start the series off with an article from Drs. Surbhi Shah and Ronald Go, who provide a detailed overview of cold agglutinin disease, an uncommon but serious disorder that can be a challenging diagnosis, and the potential treatment options. Thankfully, therapeutic advances have finally caught up with these disorders and have seen tremendous progress over the past few years, such as gene therapy for sickle cell disease. We anticipate that these advances will one day make them truly benign, but much work needs to be done to get there.
Cold Agglutinin Disease
Cold agglutinin disease (CAD) was originally described by Helmut Schubothe in 1966 as a rare disorder that is mediated by cold antibodies causing complement-dependent immune hemolysis.1 This disorder contributes to 10 to 15% of autoimmune hemolytic anemia (AIHA) cases.2
Patients can have primary CAD in the context of immunoglobulin M (IgM) monoclonal protein in the serum; recently it has been characterized as a distinct clonal B-cell lymphoproliferative disorder.3 Secondary form, or cold agglutinin syndrome (CAS), is typically associated with an infection such as Mycoplasma pneumoniae or Epstein-Barr virus.4, 5
Pathophysiology
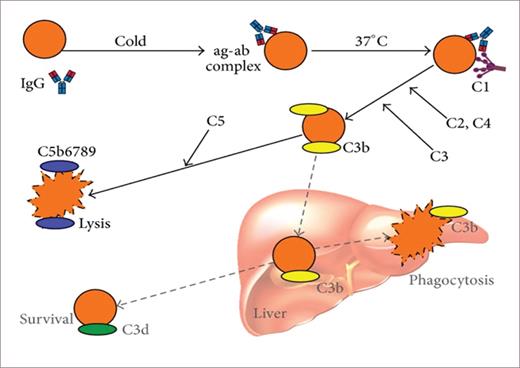
CAD is mediated by monoclonal IgM immunoglobulin with kappa light chain restriction in 90% of cases.6 The IgM remains unbound from the red cell surface when the blood circulates in the body core but as it makes its way toward the peripheral circulation, particularly in acral areas, IgM transiently binds to the I antigen binding site on the red cell membrane. It then fixes the complement on the surface of the red cell, activating the complement cascade (C3 binding). Upon the return of blood to the core of the body, IgM dissociates and the C3b-coated cell is subsequently phagocytosed by receptor-specific macrophages predominantly present in the liver. This process leads to extravascular hemolysis.7, 8 (Figure 1)
Immune-initiated, complement-mediated erythrocyte destruction in cold agglutinin disease (CAD) and cold agglutinin syndrome (CAS).
Berentsen S, et al. Biomed Res Int. 2015;2015:363278.
Immune-initiated, complement-mediated erythrocyte destruction in cold agglutinin disease (CAD) and cold agglutinin syndrome (CAS).
Berentsen S, et al. Biomed Res Int. 2015;2015:363278.
Clinical Presentation
Patients with CAD typically present in their 70s, with a median age around 67 years. Females have a higher propensity of developing this disorder.6, 9 The most common presentation is anemia, with hemoglobin ranging from 4 to 10 g/dL. Severe anemia is present in approximately one-third to one-fourth of patients.10
The diagnosis is typically made secondary to symptoms of hemolytic anemia such as fatigue or cold-induced agglutination in the capillary circulation, which causes acrocyanosis, livedo reticularis, livedo racemosa, or even disabling Raynaud’s phenomenon.6 Patients with CAD can develop life-threatening hemolysis in the setting of febrile infectious complications or surgical intervention, both of which can exacerbate hemolysis.11, 12
Diagnosis
The hallmark of CAD is agglutination of red cells on a blood smear. Biochemical evidence of hemolysis, with a direct antiglobulin test strongly positive for complement C3d and a cold agglutinin titer above 64, clinches the diagnosis of CAD.13
Laboratory results that help with the diagnosis of CAD are a complete blood count, which demonstrates varying degrees of anemia with elevated mean corpuscular volume (this can be related to reactive reticulocytosis or agglutination), reticulocyte count, lactate dehydrogenase, total and indirect bilirubin, and low haptoglobin. A direct antiglobulin test is the key to a CAD diagnosis; the test will be strongly positive for C3 and also positive for immunoglobulin G (IgG) in up to 20% of cases. In most cases, serum electrophoresis with immunofixation will show monoclonal IgM kappa class antibodies. Given the temperature sensitivity of the antibodies, the processing and handling of specimens becomes critically important.14
B-cell clonality has been highlighted by several researchers as a peculiar clonal rearrangement of the heavy or light chain genes; this peculiarity differentiates this clonal B-cell process from what was then thought to be lymphoplasmacytic lymphoma (LPL) or Waldenström macroglobulinemia. Furthermore, the most prominent feature of CAD is absence of the MYD88 L265P mutation, which is seen in 90% cases of LPL. This mutation could not be demonstrated in CAD-associated lymphoproliferative disease. Clonal rearrangement of IGKV3-20 gene sequences in highly homogeneous CDR3 regions is seen in more than half of cases. Recurrent mutations in KMT2D and CARD11 were also seen.15
Further evaluation is necessary to rule out CAS secondary to infectious etiology and to determine if any monoclonal protein is detected.16
Treatment
In the past, pharmacotherapy for CAD was reserved for patients with severe hemolysis due to poor response to the available therapeutic options such as steroids,17 azathioprine,6 and interferon.18 Cold avoidance and prompt treatment of infections, as well as warmed transfusions, were the cornerstones of therapy for patients with compensated low-grade hemolysis.19
With significant advances in pharmacotherapy in past decades, patients with symptomatic mild-to-moderate anemia or cold-induced circulatory symptoms should be considered for therapy. Recombinant erythropoietin can be used for supportive therapy.20
B-cell–directed chemoimmunotherapy is the cornerstone for management of primary CAD. Table 1 describes the choices and outcomes.
B-cell–directed chemo-immunotherapy.
| Initial/relapse setting . | Phase III? . | N . | Overall response rate . | Median time to response . | Median duration of response . | FDA approval? . |
|---|---|---|---|---|---|---|
| Rituximab13 | No | 20-32 | 45-54% | 2 months | 10 months | No |
| Rituximab + prednisone24 | No | 9 | 56% | 0.5 months | N/A | No |
| Rituximab + fludarabine25 | No | 29 | 76% | 4 months | >66 months | No |
| Rituximab + bendamustine26 | No | 45 | 71% | 1.9 months | >88 months | No |
| Chlorambucil6, 13 | No | 19-37 | 16-46% | N/A | 11 months | No |
| Bortezomib27 | No | 19 | 32% | N/A | 16 months | No |
| Ibrutinib28 | No | 10 | 100% | 1 month | 9 months | No |
| Initial/relapse setting . | Phase III? . | N . | Overall response rate . | Median time to response . | Median duration of response . | FDA approval? . |
|---|---|---|---|---|---|---|
| Rituximab13 | No | 20-32 | 45-54% | 2 months | 10 months | No |
| Rituximab + prednisone24 | No | 9 | 56% | 0.5 months | N/A | No |
| Rituximab + fludarabine25 | No | 29 | 76% | 4 months | >66 months | No |
| Rituximab + bendamustine26 | No | 45 | 71% | 1.9 months | >88 months | No |
| Chlorambucil6, 13 | No | 19-37 | 16-46% | N/A | 11 months | No |
| Bortezomib27 | No | 19 | 32% | N/A | 16 months | No |
| Ibrutinib28 | No | 10 | 100% | 1 month | 9 months | No |
However, slow-to-achieve and suboptimal responses to chemoimmunotherapy (RR of 50 to 76%) led to the investigation of whether complement modulation could help achieve faster response rates (Table 2).
Complement-directed therapy.
| Initial/relapse setting . | Phase III? . | N . | Overall response rate . | Time to median response . | Median duration of response . | FDA approval? . |
|---|---|---|---|---|---|---|
| Eculizumab29 | No | 13 | 54% | N/A | NA | No |
| Sutimlimab21 | Yes | 22 | 73% | 0.3 months | NA | Yes |
Sutimlimab is a humanized monoclonal antibody against C1 and the first agent approved by the U.S. Food and Drug Administration (FDA) for the management of CAD. Röth and colleagues published results from the open-label, single-arm, phase III CARDINAL study where sutimlimab demonstrated more rapid clinical and laboratory improvements in comparison to placebo.21 This study was followed by the randomized, placebo-controlled phase III CADENZA trial, in which sutimlimab demonstrated reduced hemolysis, anemia, and fatigue compared with placebo, with minimal adverse events.22 Unfortunately, the response to the drug is limited to the duration of therapy and it does not improve the symptoms related to Raynaud’s phenomenon.23
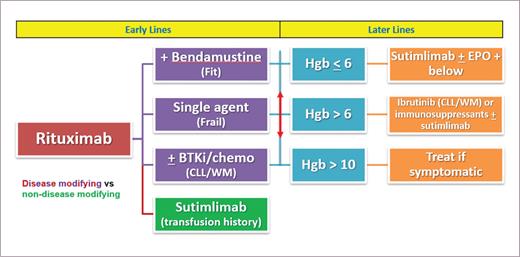
Figure 2 outlines our approach to management of CAD.
Future Directions
Other agents are under investigation, such as ANX005, a humanized monoclonal antibody that inhibits C1q, which is being evaluated in a safety trial with healthy volunteers (ClinicalTrials.gov: NCT03010046). APL-2 is a compstatin-based pegylated cyclic peptide inhibitor of C3; it prevents the formation of C3b. It is currently under evaluation for safety, tolerability, and efficacy in patients with warm autoimmune hemolytic anemia (AIHA) or CAD (ClinicalTrials.gov: NCT03226678).
Conclusion
CAD is a complement-driven rare AIHA with an underlying B-cell clonal disorder. B-cell–directed chemoimmunotherapy leads to slow-to-achieve responses but can offer long-term remissions. With the recent FDA approval of complement-directed therapy, prompt improvement is made possible. These options have opened the conversation for sequencing or combining these therapies. It might be pragmatic to combine both therapies, where complement-directed therapy helps in early control of hemolysis while the immune-ablative effect of B-cell– directed therapy is awaited. Further studies are needed to assess this approach.
Competing Interests
Dr. Shah and Dr. Go indicated no relevant conflicts of interest.