I have been looking after children with sickle cell disease (SCD) in the U.K. for more than 20 years, and the choice of therapeutic options has not changed substantially during that period: penicillin, hydroxyurea, blood transfusions, and hematopoietic cell transplantation.1 Despite this, many outcomes have improved, because of better advice to families, more effective primary stroke prevention, and widespread use of hydroxyurea at higher doses. More drugs are beginning to emerge, notably L-glutamine, voxelotor, and crizanlizumab, although these are at best moderately effective, and none are available for the treatment of younger children in Europe nor affordable in most parts of the world.2 Over the same period, there has been an explosion of drugs in many other areas of medicine, with more than 100 drugs licensed for the different types of leukemia.3 Even very rare conditions have seen the development of effective drugs; for example, three drugs are licensed to treat paroxysmal nocturnal hemoglobinuria, with several others in development.
The lack of progress is perhaps more surprising given that the molecular basis of SCD has been understood for more than 70 years,4 and is, in theory at least, fairly simple: deoxygenated hemoglobin S (HbS) polymerizes and damages the red cell, causing vasoocclusion and hemolysis, leading to numerous downstream pathological events.5 A corollary of this is that complete inhibition of HbS polymerization would effectively cure the condition.6 There are many reasons why producing a specific inhibitor of HbS polymerization has not happened; it is technically very difficult and there has been a relative lack of funding for research into SCD in both commercial and noncommercial settings. The reasons for this lack of funding are complicated, but are undoubtedly related to the fact that SCD predominantly affects non-white populations and is most common in low-income countries.
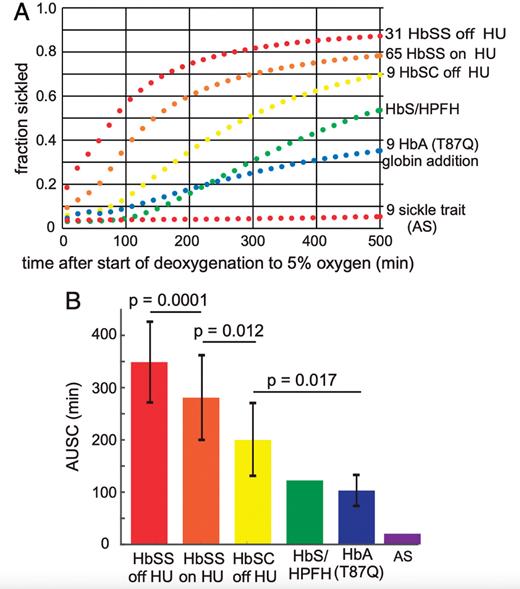
In this paper, Metaferia and colleagues describe a novel way to screen drugs for their ability to inhibit sickling, the change in red cell shape caused by HbS polymerization. They exposed sickle trait erythrocytes to 100% nitrogen and recorded shape changes in 100 to 300 cells using an automated microscopy system (Figure). Drugs were screened in 384 well plates, allowing a fairly high throughput of assays. The use of sickle trait cells is perhaps counterintuitive but gives a number of advantages over using sickle cell anemia erythrocytes, including a much greater delay before polymerization occurs, more pronounced red cell distortion, and greater cell homogeneity. The authors screened more than 12,000 compounds from the Scripps ReFRAME library of drugs already approved by the U.S. Food and Drug Administration or previously tested in clinical trials. They identified 106 drugs as having anti-sickling properties, with 21 showing significant activity at low concentrations (<10 μM). The actions of some of these drugs have been confirmed in further experiments, including studies looking at the effects on sickle cell anemia cells.
In Brief
This paper has identified at least 20 drugs, all of which are currently available for human trials and have the potential to inhibit red cell sickling at achievable concentrations. These drugs may act through direct inhibition of HbS polymerization, changes in oxygen binding to hemoglobin, or possibly on the pathways responsible for red cell dehydration and membrane damage, which ultimately cause the red cell to change shape and sickle.7 Inhibition of red cell sickling implies a reduction in vaso-occlusion and hemolysis, which may translate into clinical benefit. The ReFRAME drug library is particularly appealing because all the drugs are ready for human use, but there are many other libraries containing hundreds of thousands of other compounds which could potentially be screened in the same way. Hopefully, these efforts will lead to the eventual development of a wide range of effective, affordable drugs which allow precision medicine to become a reality for people with SCD.8
Competing Interests
Dr. Rees indicated no relevant conflicts of interest.