STUDY TITLE: The Pediatric Acute Leukemia (PedAL) Screening Trial - A Study to Test Bone Marrow and Blood in Children with Leukemia That Has Come Back After Treatment or Is Difficult to Treat - A Leukemia & Lymphoma Society and Children’s Oncology Group Study
CLINICAL TRIALS.GOV IDENTIFIER: NCT04726241
PARTICIPATING CENTERS: ~220 institutions in the United States, Canada, Australia, and New Zealand
SPONSOR: The Leukemia & Lymphoma Society (LLS) PedAL Initiative in collaboration with the Children’s Oncology Group and the National Cancer Institute
ACCRUAL GOAL: 960 children, adolescents, and young adults younger than 22 years of age
STUDY DESIGN: The APAL2020SC master trial is a registry and screening study sponsored by The Leukemia & Lymphoma Society (LLS) Pediatric Acute Leukemia (PedAL) Initiative. The parallel European Pediatric Acute Leukemia (EuPAL) initiative sponsors the EuPAL 2021 Registry, which will collect data similar to those in the APAL2020SC registry.1
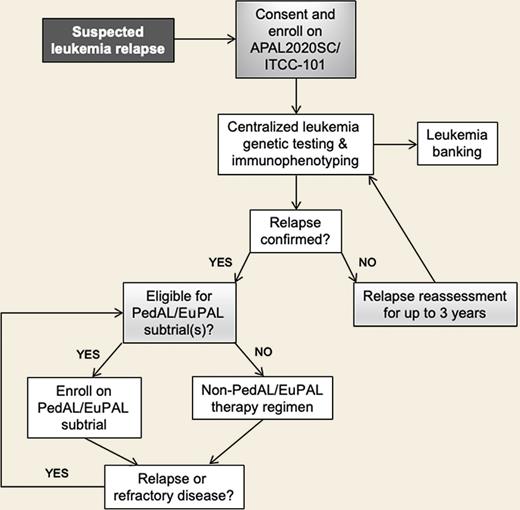
The international collaborative PedAL/EuPAL effort aims to perform detailed cytomolecular and immunophenotypic profiling of relapsed pediatric and adolescent/young adult acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and mixed phenotype acute leukemia (MPAL) specimens. This information may help patients, families, and treating physicians allocate patients to biologically relevant precision medicine–based clinical trials designed to investigate the safety, feasibility, and/or efficacy of targeted therapies with or without combination chemotherapy. APAL2020SC also serves as a biorepository for banked relapsed/refractory leukemia specimens and as a mechanism for longitudinal tracking of patients following relapse (Figure). Participating consortia include the Children’s Oncology Group (COG) and the Australian and New Zealand Children’s Haematology/Oncology Group (ANZCHOG).1
Historically, access to novel agents for children with relapsed/refractory leukemia has been quite poor due to the relative rarity of pediatric versus adult cancers, hypothetical a priori safety concerns, potential lack of profitability for pharmaceutical companies, and a misperceived need to wait for mature adult data on new drugs before testing them in children.2-4 The LLS PedAL Initiative aims to overcome the latter barrier by expediting development of new targeted therapies for challenging-to-treat childhood leukemias and potentially replacing one-size-fits-all chemotherapy with treatments tailored to each child’s unique tumor biology.
The recently opened international ITCC-101/APAL2020D phase III subtrial (NCT05183035) is evaluating the overall and event-free survival of children with relapsed/refractory AML. Participants are randomized to fludarabine/cytarabine chemotherapy with filgrastim (g-csf) support (FLAG) or FLAG combined with the BCL-2 inhibitor venetoclax.5 Additional soon-to-open or in-development PedAL/EuPAL sub-trials will explore e-selectin ligand inhibition, menin inhibition, CD47 inhibition, CD123-targeted immunotherapies, and other targeted immunotherapies.
RATIONALE: Critical oncogenic driver mutations and protein targets identified in high-risk acute leukemias provide major opportunities for the development of precision therapeutics to improve clinical outcomes. Hallmark examples include antibody-based and cellular CD19- or CD22-targeting immunotherapies, which have demonstrated remarkable efficacy in patients with relapsed/refractory B-cell ALL and facilitated multiple approvals by the U.S. Food and Drug Administration and European Medicines Agency. B-ALL is the most common childhood and adolescent/young adult cancer and has near-universal expression of CD19 and CD22 antigens,6,7 which facilitated these achievements and enabled study of these immunotherapies in traditionally designed and enrolled trials. However, studying novel therapies becomes difficult in increasingly smaller “boutique” subtypes of high-risk leukemias, when only small numbers of patients have the necessary target for a precision medicine therapy. The difficulty of enrolling only small cohorts of patients in a wide range of phase I/II trials is compounded by the often rapidly accelerating nature of leukemia relapse, which compresses the time available to access and start a new therapy.
In the LLS Beat AML Master Clinical Trial for older adults with newly diagnosed AML, investigators performed cytomolecular leukemia characterization to allocate patients to precision medicine subtrials (NCT03013998).8 Importantly, disease profiling was completed within seven days, and patients treated in Beat AML substudies were ultimately shown to have substantially improved overall survival compared to those treated with standard-of-care therapies.9 While the biology of pediatric leukemias is demonstrably different from that of adult leukemias, Beat AML established both feasibility and efficacy for the global PedAL/EuPAL pediatric-specific precision medicine umbrella trial efforts.10
COMMENT: Uniform treatment of children with newly diagnosed AML or ALL with multi-agent chemotherapy regimens on cooperative group clinical trials has led to improved event-free and overall survival during the past 40 years and, importantly, has enabled discovery of critical genetic, immunophenotypic, and measurable residual disease biomarkers that influence prognosis.6,11 However, salvage approaches for patients with relapsed disease have been more variable with long-term remission success increasingly dependent upon timing and site of relapse and leukemia-associated genetics.12,13
A major barrier at present is that many childhood leukemia subtypes at high risk of relapse occur in such small numbers that no single institution, or even country, can accrue sufficient patient numbers to study relevant targeted therapies with any efficiency. The PedAL APAL2020SC trial and the EuPAL 2021 Registry are designed to overcome this barrier by creating a central hub in North America, Australia, and New Zealand and a central hub in Europe to follow patients with relapsed/refractory AML, ALL, and MPAL. These initiatives also aim to increase access to early-phase clinical trials of promising targeted therapies. It is hoped that these efforts will prove to be a major step forward in personalized medicine for and eradication of high-risk pediatric acute leukemias (Avada Kedavra!).14
Competing Interests
Dr. Hughes indicated no relevant conflicts of interest. Dr. Tasian serves as an uncompensated advisor for the LLS PedAL Initiative.