STUDY TITLE: A Randomized Phase II Study Comparing Inotuzumab plus Chemotherapy versus Standard Chemotherapy in Older Adults with Philadelphia-chromosome-negative B-Cell Acute Lymphoblastic Leukemia (ALLIANCE A042001)
CLINICAL TRIALS.GOV IDENTIFIER: NCT05303792
PARTICIPATING CENTERS: Alliance, ECOG, and SWOG centers
ACCRUAL GOAL: 66 patients
STUDY DESIGN: Randomized phase II open-label
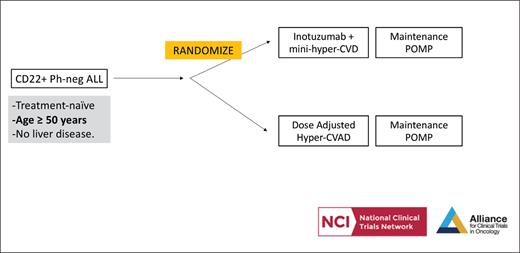
STUDY DESIGN: A042001 is a randomized phase II clinical trial being conducted by U.S. adult cancer cooperative groups to investigate whether the experimental arm — inotuzumab ozogamicin (INO) in combination with mini-hyperCVD (cyclophosphamide, vincristine, dexamethasone alternating with methotrexate, cytarabine) — results in superior measurable residual disease (MRD) –negative event-free survival (EFS) compared with a control arm of dose-adjusted hyperCVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with methotrexate, cytarabine). The study population consists of previously untreated adults aged 50 years or older with CD22+ Philadelphia chromosome–negative (Ph-) B-cell acute lymphoblastic leukemia (ALL). Secondary objectives include standard response and survival criteria, in addition to comparison of techniques to measure MRD, modified geriatric assessments, and patient-reported outcomes.
RATIONALE: The most recent five-year overall survival rate for adults 40 years or older with ALL remains below 50 percent, with estimates dropping precipitously with each decade.1 In adult ALL, the highly active, minimally toxic antibody-based therapies blinatumomab and INO have revolutionized salvage therapy,2,3 allowing many relapsed patients to achieve a second complete remission and become eligible for curative-potential hematopoietic cell transplantation. Incorporating these potent, well-tolerated novel agents into frontline adult ALL regimens is a logical next step, particularly for older adults, in whom conventional multiagent chemotherapy regimens are especially toxic and only modestly effective. Thus, the use of novel agents up-front may both improve efficacy and decrease toxicity through de-escalation of conventional chemotherapy.
Investigators at The University of Texas MD Anderson Cancer Center were among the first to incorporate novel agents into frontline therapy in adult ALL. In a single-center study they demonstrated that INO in combination with mini-hyperCVD +/- blinatumomab was safe and resulted in an impressive MRD-negative complete remission in more than 80 percent of patients, with three-year continuous complete remission duration and overall survival rates of 75 percent and 54 percent, respectively.4 In a propensity weighted analysis, INO in combination with mini-hyperCVD +/- blinatumomab was associated with higher response rates and a near doubling of survival compared with standard hyperCVAD.5 The A042001 study now seeks to confirm these findings in a multicenter prospective randomized clinical trial.
COMMENT: Although exciting clinical data have been presented with the combination of INO and reduced intensity chemotherapy, broad adoption of this regimen as the standard for newly diagnosed older adults with CD22+ B-cell ALL will be substantiated if superiority is clearly demonstrated in this randomized, multicenter trial. The original schema of the A042001 trial did not incorporate postremission blinatumomab; however, following the remarkable results of the E1910 trial showing superiority with blinatumomab consolidation in patients with MRD, an early amendment is planned to add blinatumomab cycles to both the experimental and control arms. Given the rapidity of developments occurring in the field of ALL, A042001 was aptly designed as a smaller phase II study, with the primary end point based in part on an early treatment milestone (achieving MRD negativity after 2 cycles of therapy), aiming for faster enrollment and an accelerated timeline to study readout. This is an exciting era in adult B-cell ALL — it is conceivable that novel targeted immunotherapy-based regimens will largely replace conventional chemotherapy in many treatment settings — and the results of A042001 will provide an important milestone in this therapeutic revolution.
Clinical trial schema, A042001, for newly diagnosed older adults with CD22+ Ph-negative B-cell ALL. POMP, (6-mercaptopurine, vincristine, methotrexate, prednisone).
Clinical trial schema, A042001, for newly diagnosed older adults with CD22+ Ph-negative B-cell ALL. POMP, (6-mercaptopurine, vincristine, methotrexate, prednisone).
Competing Interests
Dr. Muffly has received honoraria from Pfizer and has served on an Amgen advisory board.