T-cell–redirecting bispecific antibodies (bsAbs) and chimeric antigen receptor T cells (CAR-Ts) targeting B-cell maturation antigen (BCMA) generate astounding response rates in relapsed/refractory multiple myeloma (MM).1,2 Nonetheless, a plateau on the survival curve remains sadly elusive. While we may be pleasantly surprised as the survival curves mature, it is prudent to expect that MM might develop resistance, even against these promising new therapies. If we can understand and overcome this resistance, we may be able to increase response rates and lengthen remissions. Can a single unifying explanation for resistance be found (and targeted)? Or must we accept that this famously heterogeneous disease3 exhibits a cacophony of resistance mechanisms?
This Feature will focus on anti-BCMA bsAbs and CAR-T immunotherapy. We deliberately exclude the third class of BCMA-targeting agent — antibody-drug conjugates — whose mechanism of action includes nonimmune cytotoxicity (Table; Figure).4–10 We will address two of the most likely culprits for resistance: 1) the malignant plasma cell that defines MM, and 2) the immune system (both native and modified), and determine which is the biggest barrier to therapeutic success.
A Non-exhaustive List of B-cell Maturation Antigen–targeting Products
| Construct Name . | Class . | ORR (%) . | License . | Grade 3 Adverse Events (>10% patients) . |
|---|---|---|---|---|
| Belantamab mafodotin5 | ADC | 31 | United States and Europe | Keratopathy Thrombocytopenia Anaemia Lymphopenia |
| Teclistamab6 | bsAb | 65 | Trial only | Neutropenia Anaemia Thrombocytopenia Leukopenia (CRS, 0%) (Neurotoxicity, NR) |
| Elranatamab1 | bsAb | 75 | Trial only | Neutropenia Anaemia Thrombocytopenia Lymphopenia (CRS 0%) (Neurotoxicity, 0%) |
| Pavurutamab (AMG 701)7 | bsAb | 36 | Trial only | Most grades NR (CRS, 9%) (Neurotoxicity, 0%) |
| REGN54588,9 | bsAb | 36 | Trial only | Most grades NR Infection (CRS, NR) (Neurotoxicity, NR) |
| Idecabtagene vicleucel10 BMS | CAR-T | 85 | United States only | Neutropenia Anemia Thrombocytopenia Leukopenia Lymphopenia Febrile neutropenia (CRS, 5%) (Neurotoxicity, 3%) |
| Ciltacabtagene autoleucel2 | CAR-T | 97 | United States only | Anemia Thrombocytopenia Leukopenia Lymphopenia (CRS, 4%) (Neurotoxicity, 9%) |
| Construct Name . | Class . | ORR (%) . | License . | Grade 3 Adverse Events (>10% patients) . |
|---|---|---|---|---|
| Belantamab mafodotin5 | ADC | 31 | United States and Europe | Keratopathy Thrombocytopenia Anaemia Lymphopenia |
| Teclistamab6 | bsAb | 65 | Trial only | Neutropenia Anaemia Thrombocytopenia Leukopenia (CRS, 0%) (Neurotoxicity, NR) |
| Elranatamab1 | bsAb | 75 | Trial only | Neutropenia Anaemia Thrombocytopenia Lymphopenia (CRS 0%) (Neurotoxicity, 0%) |
| Pavurutamab (AMG 701)7 | bsAb | 36 | Trial only | Most grades NR (CRS, 9%) (Neurotoxicity, 0%) |
| REGN54588,9 | bsAb | 36 | Trial only | Most grades NR Infection (CRS, NR) (Neurotoxicity, NR) |
| Idecabtagene vicleucel10 BMS | CAR-T | 85 | United States only | Neutropenia Anemia Thrombocytopenia Leukopenia Lymphopenia Febrile neutropenia (CRS, 5%) (Neurotoxicity, 3%) |
| Ciltacabtagene autoleucel2 | CAR-T | 97 | United States only | Anemia Thrombocytopenia Leukopenia Lymphopenia (CRS, 4%) (Neurotoxicity, 9%) |
Abbreviations: ADC, antibody-drug conjugates; bsAb, bispecific antibody; CAR-T, chimeric antigen receptor T cell; CRS, cytokine release syndrome; NR, not reported.
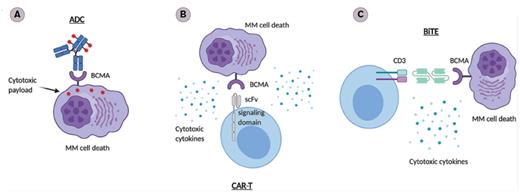
B-cell Maturation Antigen (BCMA) –targeted Immunotherapies. A) Antibody-drug conjugate (ADC): Upon binding to BCMA on the surface of multiple myeloma (MM) cells, ADC is internalized first and the linker is hydrolyzed inside of the lysosomes or endosomes, releasing the payloads that lead to cell death. B) Chimeric antigen receptor cells (CAR-Ts): The ectodomain of the BCMA scFv on the CAR-Ts binds to BCMA on the surface of MM cells. This leads to activation of the CAR-Ts, which release cytotoxic cytokines and cause MM cell death. C) Bispecific T-cell engager (BiTE). The dual BCMA- and CD3-scFv–containing BiTE binds concomitantly to CD3 and BCMA, facilitating T cell/MM cell crosslinking, followed by CD4+/CD8+ T-cell activation and secretion of cytotoxic cytokines, leading to MM cell death.
B-cell Maturation Antigen (BCMA) –targeted Immunotherapies. A) Antibody-drug conjugate (ADC): Upon binding to BCMA on the surface of multiple myeloma (MM) cells, ADC is internalized first and the linker is hydrolyzed inside of the lysosomes or endosomes, releasing the payloads that lead to cell death. B) Chimeric antigen receptor cells (CAR-Ts): The ectodomain of the BCMA scFv on the CAR-Ts binds to BCMA on the surface of MM cells. This leads to activation of the CAR-Ts, which release cytotoxic cytokines and cause MM cell death. C) Bispecific T-cell engager (BiTE). The dual BCMA- and CD3-scFv–containing BiTE binds concomitantly to CD3 and BCMA, facilitating T cell/MM cell crosslinking, followed by CD4+/CD8+ T-cell activation and secretion of cytotoxic cytokines, leading to MM cell death.
It's Probably the Plasma Cells
The subclonal evolution of malignant plasma cells is hypothesized to largely account for patient outcomes in MM, where treatment-resistant subclones become dominant and drive each inevitable relapse.11 For instance, a portion of patients treated sequentially with the immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide acquire deletions or mutations in loci encoding the IMiD target cereblon and other proteins fundamental to cereblon's processing.12,13 The frequency of these genetic disruptions increases after each IMiD exposure, strongly suggesting a treatment-specific selection pressure that promotes evolutionary escape.
This same mechanism plausibly explains relapse following BCMA-targeting therapy; after all, the selection pressures exerted by CAR-T and bsAbs are potent, as seen in B-cell acute lymphoblastic leukemia (B-ALL) and loss of CD19 expression.14 Sure enough, biallelic loss of the TNFRSF17 gene encoding BCMA has been described and correlates with resistance to BCMA-targeting CAR-T and bsAb.15–17 Even if TNFRSF17 is not lost through deletion or mutation events, mean fluorescence intensity profiles confirm the presence of a BCMA(low)– expressing plasma cell populations immediately after therapy and at relapse in several patients treated with anti-BCMA CAR-T.18
The strong selection pressures of immunotherapy might additionally promote plasma cell-intrinsic escape mechanisms downstream of BCMA. Single-cell RNA sequencing of bone marrow mononuclear cells from a patient who relapsed almost two years following anti-BCMA CAR-T therapy showed an increase in anti-apoptotic gene expression compared to baseline, a finding that was then replicated in other relapsing patients in the same cohort (and seen in non-responding patients, too).19
Except Maybe It's Not
And yet, there are clues to suggest that these tumor-intrinsic mechanisms do not always speak to the core of the resistance phenomenon. In contrast to the frequent loss of CD19 in B-ALL relapse,14 the loss of BCMA — either at the genetic or protein level — is patchy. A subanalysis of patients treated with idecabtagene vicleucel (ide-cel, the first anti-BCMA CAR-T product to be licensed), found only one of 16 evaluable patients had lost BCMA expression at relapse.10 Similar observations have been made elsewhere,19,20 and critically, re-treatment with BCMA-targeting therapy can be successful.1 Indeed, 97 percent of patients relapsing after ide-cel had rising serum BCMA levels,10 which suggests that for this cohort at least, BCMA(low) plasma cells are unlikely to be the reservoir for resistance. More broadly, gene expression changes such as increased anti-apoptotic gene expression may reflect a dynamic state consequent to an exogenous resistance signal, rather than the cause of resistance itself.
So Perhaps It's the Immune System Then?
It is certainly possible to argue that the immune system is critical in controlling MM and that by extension, unfavorable immune activity contributes significantly to relapse. Its anti-tumor role suggested by immunosurveillance in patients with monoclonal gammopathy of uncertain significance (MGUS, the precursor state to MM)21 is observed in the overall survival benefit provided by allogeneic stem cell transplantation above that of autologous transplant (despite the former's higher treatment-related mortality)22 and reflected in the efficacy of established MM treatments, daratumumab and the IMiDs, whose pleiotropic effects include enhanced immune activity. The power of the immune system against MM is equally apparent when it is compromised, observed for example in the senescence of T cells that constitute the native immune response to myeloma,23 the loss of naïve and early memory T cells in relapsed disease,24 and in the profoundly immunosuppressive activity of T regulatory cells (Tregs) in murine models.25
Our understanding is incomplete, but we already have insight into immune factors that influence CAR-T activity: the presence of early memory cells in the product,26 the ratio of CD4:CD8 cells,27 and the total expansion of T cells27 are all significantly related to efficacy. Equally, bsAb activity correlates with immune parameters: the repertoire of native T cells present in the bone marrow prior to therapy determines patient response to therapy,28 while the degree of MM cell killing may depend on the manner in which the native T cells are activated.29 Regardless of whether the patient has received bsAb or CAR-T therapy, single-cell profiling of bone marrow T cells is predictive of response to therapy and reveals increased T-cell exhaustion, reduced early memory T cells, and a low proportion of CD4+ T cells in resistant/relapsed patients.20
Concluding Thoughts
There is no single, unifying explanation for resistance to BCMA-targeting therapy, primarily because of MM's extraordinary degree of inter- and intra-patient genetic heterogeneity. Nonetheless, a small number of dominant mechanisms might be envisaged which, if proven, would lead to individualized yet deliverable strategies to potentiate anti-BCMA treatments. Given the early findings of dysregulated immune cells in resistant patients, a fascinating avenue for study will be the fate of T-cell clones over time and the mechanisms by which they communicate with tumor cells — in the hope that this will reveal targets for intervention.
Competing Interests
Dr. Watson indicated no relevant conflicts of interest. Dr. Gooding receives research funding from Bristol Myers Squibb. Dr. Ramasamy receives research grants, is on the advisory boards, and receives speaker's honoraria from Bristol Myers Squibb, Janssen, and GlaxoSmithKline.