Study Title: A Phase 2 Randomized Study Comparing Venetoclax and Azacitidine to Induction Chemotherapy for Newly Diagnosed Fit Adults With Acute Myeloid Leukemia
ClinicalTrials.gov Identifier: NCT04801797
Participating Centers: Massachusetts General Hospital; Beth Israel Deaconess Medical Center
Accrual Goal: Approximately 172 participants
Sponsor: AbbVie, Inc.
Study Design: This study is a randomized phase II trial comparing current standard-of-care intensive cytarabine-based induction chemotherapy (IC) to the combination of the BCL-2 inhibitor venetoclax and hypomethylating agent (HMA) azacitidine (HMA/ven). It is enrolling adults 18 years and older who have newly diagnosed, untreated acute myeloid leukemia (AML) and are deemed fit for traditional cytotoxic induction chemotherapy per the treating physician. Patients must have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 or less, left ventricular ejection fraction of 50 percent or higher, and adequate hepatic function. Patients with secondary AML can be enrolled but cannot have previously received a hematopoietic stem cell transplant (HCT) for a myeloid neoplasm or be at risk of exceeding their lifetime anthracycline exposure limit in the trial. Those randomized to IC receive either seven days of continuous-infusion cytarabine with three days of anthracycline (“7+3”), or for patients with secondary disease, liposomal daunorubicin and cytarabine (CPX-351) for induction followed by consolidation. Those randomized to HMA/ven receive azacitidine on days 1 to 7 and venetoclax on days 1 to 28 for up to three 28-day cycles, which can be extended if there is a response. Patients can proceed to HCT after either therapy if deemed appropriate by the treating clinician.
The primary objectives of the study are to compare the event-free survival of patients treated with IC versus HMA/ven. Secondary objectives include evaluating the rate of response, measurable residual disease (MRD), treatment-related toxicity, overall survival (OS), HCT rate, health care utilization metrics (such as number of hospitalizations), and patient-reported outcomes including quality of life.
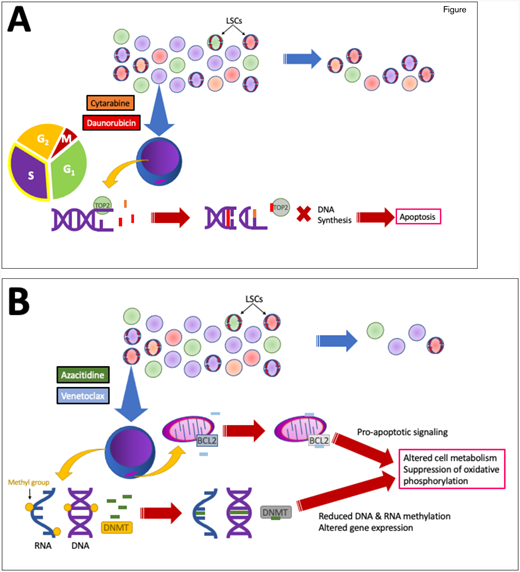
Rationale: Since the U.S. Food and Drug Administration (FDA) gave accelerated approval of venetoclax for treating AML in 2018, and subsequent full approval based on the results of the VIALE-A study,1 combination HMA/ven therapy has become the frontline standard of care for adults 75 years or older with AML, or for those deemed to be too unfit to receive standard IC regardless of age. However, the standard of care for younger, fit adults with newly diagnosed AML remains conventional IC regimens. Mechanistically, these two regimens differ — while IC broadly impacts leukemic blasts (and other rapidly dividing cells) by interfering with DNA replication, HMA/ven is hypothesized to specifically target leukemic stem cell metabolism by affecting oxidative phosphorylation (Figure).2
A. Cytarabine (orange) and anthracyclines such as daunorubicin (red) and idarubicin work in concert to prevent DNA synthesis in rapidly dividing cells, including leukemic blasts, thereby leading to cell death. However, chemoresistant subclones (red, orange, green circles) may persist, particularly quiescent leukemic stem cells (LSCs; shown with red circular arrows). B. Azacitidine (green) has long been used as a monotherapy to alter leukemic cell gene expression by incorporating into both DNA and RNA, inhibiting methyltransferases, and reducing nucleic acid methylation. Venetoclax (light blue) inhibits the anti-apoptotic protein BCL-2, which resides in the outer mitochondrial membrane. Combined, they appear to disproportionately disrupt LSC metabolism due to dependence on oxidative phosphorylation, potentially targeting the LSCs more effectively. TOP2, topoisomerase 2; DNMT, DNA methyltransferase.
A. Cytarabine (orange) and anthracyclines such as daunorubicin (red) and idarubicin work in concert to prevent DNA synthesis in rapidly dividing cells, including leukemic blasts, thereby leading to cell death. However, chemoresistant subclones (red, orange, green circles) may persist, particularly quiescent leukemic stem cells (LSCs; shown with red circular arrows). B. Azacitidine (green) has long been used as a monotherapy to alter leukemic cell gene expression by incorporating into both DNA and RNA, inhibiting methyltransferases, and reducing nucleic acid methylation. Venetoclax (light blue) inhibits the anti-apoptotic protein BCL-2, which resides in the outer mitochondrial membrane. Combined, they appear to disproportionately disrupt LSC metabolism due to dependence on oxidative phosphorylation, potentially targeting the LSCs more effectively. TOP2, topoisomerase 2; DNMT, DNA methyltransferase.
Though tools have been developed to assess geriatric fitness,3,4 specifically for IC in older patients with AML,5,6 fitness assessment is often subject to physician bias.7 In fact, more than half of the patients in the VIALE-A trial had an ECOG PS of 1 or less, and 40 percent were younger than 75 years.1 Given the durable responses seen with HMA/ven in multiple trials,1,8,9 younger and fit patients outside of the original target population for HMA/ven may also benefit from this therapy in the upfront setting.
Importantly, several retrospective studies have failed to definitively determine whether younger and fit patients with AML may benefit from HMA/ven over IC. Dr. Evan Cherry and colleagues performed a retrospective, single-institution review of a modestly sized (n=359) patient population treated with either IC or HMA/ven.10 Patients treated with HMA/ven were significantly older than those treated with IC (69.5 vs. 52.7 years; p<0.001) and more often had adverse European LeukemiaNet (ELN) risk (65.0% vs. 40.3%; p<0.0001). Though median OS was significantly longer for patients treated with IC (884 days) compared to HMA/ven (483 days; p=0.002), median OS appeared inferior in IC (483 days) versus HMA/ven (not reached; p=0.0667) when matching for patient age, ELN risk, and use of HCT. Older age, secondary AML, and RUNX1 mutation appeared to favor response to HMA/ven, whereas FAB M5 AML type favored response to IC.
Another retrospective study by Dr. Andrew Matthews and colleagues examined data pooled from a single academic institution and a multicenter real-world dataset (n=659) to look specifically at outcomes among patients who received either HMA/ven or CPX-351.11 Again, patients receiving HMA/ven were overall older than those who received CPX-351 (75 vs. 65 years). Both groups had similar median OS (13 months for CPX-351 vs. 11 for HMA/ven), and no covariates impacted OS on multivariate analysis. Infectious complications were more abundant and hospitalizations longer among patients who received CPX-351.
Comment: This trial could potentially shift the paradigm by which fit, younger adults with newly diagnosed AML are treated for the first time since 7+3 became standard of care in 1973. It will also better define the value of MRD negativity in patients receiving these therapies, which appears to be informative irrespective of the treatment intensity.12,13 Notably, it excludes patients with FLT3-ITD or FLT3-TKD mutations. It also excludes patients who previously received HMA-based therapy for antecedent disease, although these patients may still benefit from HMA/ven over IC.14 Finally, some patients with favorable risk disease, including those with core-binding factor translocations and patients younger than 60 years with NPM1-mutated AML, are excluded. Prior studies have shown that older patients with favorable-risk disease may have better outcomes with HMA/ven-based therapy than with IC.15,16
As this is a randomized trial, all patient characteristics in each treatment arm should be roughly equal; this study should not be subject to the same assignment biases seen in retrospective studies that attempted to compare IC to HMA/ven. With no upper age limit, a potential concern is that older patients will be more likely to be referred and enrolled, so hopefully there will be adequate recruitment of younger patients. Additionally, the study will likely not be powered to draw conclusions about which therapy most benefits specific AML subtypes due to its target enrollment of only 172 patients. Ultimately, we anticipate this study will help inform clinical practice in selecting upfront therapies for patients with AML of all ages and levels of fitness.
Competing Interests
Dr. Hochman and Dr. Hasserjian indicated no relevant conflicts of interest.