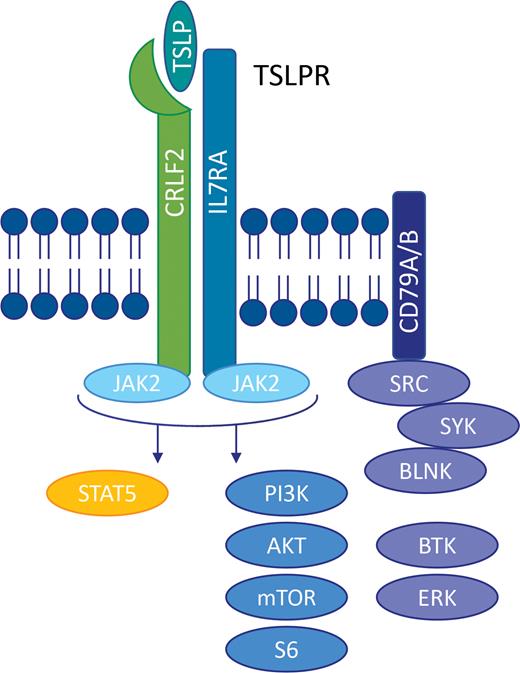
Philadelphia chromosome–like acute lymphoblastic leukemia (Ph-like ALL) is a high-risk subtype of B-cell ALL (B-ALL) occurring in 15 to 30 percent of older children, adolescents, and adults and is associated with chemoresistance, high relapse risk, and inferior long-term survival with conventional chemotherapy.1,2 Rearrangements in cytokine receptor–like factor 2 (CRLF2) comprise approximately half of Ph-like ALL cases and induce constitutive JAK/STAT and other kinase signaling that may be targetable with tyrosine kinase inhibitor–based therapies (Figure).3-7 CRLF2-rearranged Ph-like ALL is now known to be more common in patients of Hispanic/Latinx and Native American ancestry, potentially in part due to increased frequency of germline GATA3 rs3824662 variants in these populations that may also be associated with aberrant adipocyte metabolism.8-10
In this study, Dr. Steven D. Mittelman and colleagues at the Children's Hospital of Los Angeles assessed body composition metrics of fat mass and body fat via dual-energy x-ray absorptiometry scans (DEXA) of 97 children and adolescents/young adults (AYAs) with newly diagnosed National Cancer Institute high-risk B-ALL. Patients were classified as non-obese or obese based on population-norm body mass indices less than the 95th percentile or greater than or equal to the 95th percentile, respectively. The investigators also characterized diagnostic bone marrow or peripheral blood leukemia specimens via flow cytometric immunophenotyping and via fluorescence in situ hybridization, chromosomal microarray, and/or next-generation sequencing assays. Amongst the 70 patients for whom sufficient leukemia samples were available for genetic testing completion, CRLF2 rearrangements and/or increased cell surface staining of the thymic stromal lymphopoietin receptor (TSLPR, encoded by CRLF2) were detected in 23 patients (33%). Patients with CRLF2-rearranged ALL were noted to have significantly greater fat indices and rates of obesity in comparison to those with non–CRLF2-rearanged ALL (16/23 [70%] vs. 15/47 [32%]; p<0.003). The authors reported that Hispanic/Latinx ethnicity was also associated with a higher rate of obesity with specific enrichment of CRLF2 rearrangements in this cohort. Multivariable analyses further showed a 13 percent increased risk of CRLF2-R ALL for every kilogram of fat mass as measured by DEXA across the entire study population.
This report is the first to link obesity in children and AYAs with an increased incidence of a specific high-risk genetic subtype of B-ALL. The further apparent association of somatic leukemia-associated CRLF2 rearrangements with Hispanic/Latinx ethnicity and their potential interaction with obesity status is novel and requires further investigation given the relatively small cohort size in which most studied patients (54/70, 77%) were Hispanic/Latinx. The authors posit that their observed increased obesity rates in patients with CRLF2-rearranged ALL could be in part attributable to frequent GATA3 “risk alleles” that may alter adipogenesis or that increased fat mass may upregulate PI3K/mTOR signaling and cellular metabolism and contribute to Ph-like leukemogenesis and maintenance. Unfortunately, this study did not report GATA3 genotyping data or PI3K/mTOR signaling activation status of the studied patients, which could be assessed in future analyses. It would be interesting to ascertain in future studies whether the associations of obesity and CRLF2 rearrangements in patients with presumably Ph-like ALL discovered by the investigators are applicable to children with trisomy 21 (Down syndrome)-associated ALL, who also have a very high frequency of CRLF2 rearrangements3,4,11 and a tendency to higher fat mass indices.12
In Brief
Given the authors’ findings in this study, the overarching next question is whether nurture can overcome nature. It seems plausible that a deliberate focus upon obesity reduction via healthy dietary choices and increased physical activity/exercise could potentially modify genetic predisposition to CRLF2-rearranged Ph-like ALL and/or improve chemosensitivity. Indeed, the authors recently demonstrated in their single-institution prospective non-randomized Improving Diet and Exercise in ALL (IDEAL) pilot trial that caloric and nutrient restriction from diet and exercise in children and AYAs with B-ALL (not CRLF2-specific) could decrease fat mass gain during steroid-based induction chemotherapy and was associated with significantly lower end-induction minimal residual disease,13 which remains the most important prognostic biomarker in childhood B-ALL.14,15 The recently opened successor multi-site randomized IDEAL-2 study via the Therapeutic Advances in Childhood Leukemia consortium is currently assessing the effects of comprehensive exercise and dietary intervention versus standard-of-care practices on clinical outcomes in a larger cohort of pediatric patients with high-risk B-ALL (Study Chair Dr. Etan Orgel, personal communication) and will likely shed further light upon this important issue.
Competing Interests
Dr. Tasian receives research funding from Incyte Corporation for Ph-like ALL studies.