Patients with cancer have disproportionately adverse outcomes after COVID-19 infection, experiencing more severe complications from infection and also significant interruptions to delivery of standard clinical care.1 Vaccination strategies against the SARS-CoV-2 virus are highly efficacious in protection against severe complications of disease; however, SARS-CoV-2 vaccine efficacy trials have excluded patients with cancer.2–5 Recently, numerous important cohort studies have examined mRNA vaccine regimens in patients with blood cancers, specifically asking whether immunogenicity is retained in cancer patients assessed by the evaluation of qualitative or quantitative antibody response to the SARS-CoV-2 spike protein. This work extrapolates from early findings demonstrating that vaccine efficacy is linked to antibody neutralization titer.6 Importantly, while these studies rely on humoral responses to vaccination, there is likely to be a contribution from other arms of the immune system — a question for future research.
In aggregate, there are three main messages from these articles. The first is that as a cohort, patients with hematologic cancer have lower rates of anti-spike IgG seropositivity and lower titers when compared to a solid organ cancer population and non-cancer controls. In these instances, the rate of seropositivity was 85 percent versus 98 percent versus 100 percent, respectively. Although this was not examined in a randomized manner, the highest titers of vaccine responses were seen after mRNA1273 (Moderna), followed by BNT162b2 (BioNTech/Pfizer), followed by a single dose of Ad26.COV2.S (Janssen). This comparison needs to be examined by prospective studies. Interestingly, patients who previously had documented COVID infection seemed to have higher antibody titers, supporting a role for immune priming prior to vaccination.
The second common theme was that there are several specific diseases where spike antibody seroconversion and titers are most impaired. These included non-Hodgkin lymphoma, multiple myeloma, and chronic lymphocytic leukemia (CLL) — all B-cell malignancies with high rates of hypogammaglobulinemia and impaired humoral immune responses. Specifically, antibodies to SARS-CoV-2 spike protein were detected in 40 percent (66/167) of patients with CLL after two doses of mRNA vaccine.7 This result mirrors the findings of another study where just 64 percent (417/650) of patients with CLL demonstrated seropositivity after two doses of mRNA vaccine. In the large cohort observed by Dr. Lee M. Greenberger and colleagues, patients with B-cell non-Hodgkin lymphoma had the highest risk of seronegativity. Finally, in two cohorts of patients with multiple myeloma, spike-protein antibodies were detected after two doses of vaccine in 84 percent (219/260) and 95 percent (175/184) of assessed patients. Conversely, seropositivity was seen in high rates for some conditions, including Hodgkin lymphoma (99%; 64/65), chronic myeloid leukemia (97%; 33/34), acute myeloid leukemia (91%; 31/34), and acute lymphoblastic leukemia (88%; 15/17).
The final common theme is that the choice of cancer treatment can have a profound impact on the rates of seroconversion. Seropositive rates were particularly low in patients treated with monoclonal antibodies that deplete B cells (rituximab 44%; 84/191), Bruton tyrosine kinase inhibitors (BTKi; zanubrutinib 50%, n=4; acalabrutinib 43%, n=56; ibrutinib 49%, n=222), and CD19 chimeric antigen receptor T-cell therapy (14%, n=7). In a CLL cohort, none of the 22 patients that received anti-CD20 therapy in the 12 months prior to vaccination had a detectable serological response. Of those with at least 12 months between anti-CD20 therapy and vaccination, 46 percent (25/55) demonstrated serological response. These rates compare to 100 percent seropositivity in an immunocompetent population. These findings may have substantial implications for patients on maintenance B-cell–depleting therapy and therefore need careful reassessment of the perceived risk versus benefit of these strategies during this current pandemic.
These variable humoral responses to vaccination raise the question as to what more can be done. Avoidance of exposure remains a top priority in these high-risk populations. Regulators in the United States and other countries have recommended an additional booster in some immunocompromised groups per the Centers for Disease Control and Prevention. This recommendation follows data demonstrating improved serological response to third doses of mRNA vaccine in solid organ transplant recipients and renal dialysis patients.7-9 Data such as these are eagerly awaited in other immunocompromised cohorts. Pre-print data in hematological cancer cohorts reported ongoing seronegativity in those who failed to respond after a two-dose mRNA regimen, despite a third mRNA booster.10 Another potential consideration may be heterologous boosting, or the use of a second distinct vaccine stimulus to augment the initial vaccine. Along these lines, a patient actively receiving B-cell–targeting therapy (prior anti-CD20, followed by current BTKi therapy) failed to produce detectable anti-SARS-CoV-2 spike IgG after two doses of mRNA vaccine, but then produced detectable (but low titer) antibodies after boosting with Ad26.COV2.S viral vector vaccine.11
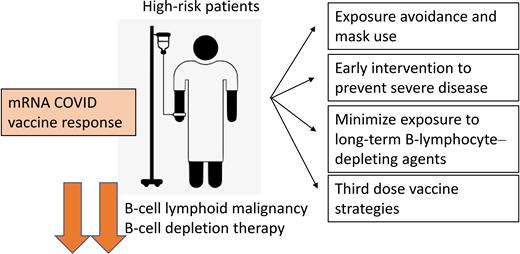
High-risk patients and mRNA COVID vaccine response. Patients with B-cell malignancies and who have had chronic B-cell–depleting therapies have reduced response to mRNA COVID-19 vaccines. Strategies to protect these patients include rigorous public exposure avoidance, early intervention to try and reduce disease severity, minimizing exposure to chronic B-cell depletion, and third dose vaccine strategies.
High-risk patients and mRNA COVID vaccine response. Patients with B-cell malignancies and who have had chronic B-cell–depleting therapies have reduced response to mRNA COVID-19 vaccines. Strategies to protect these patients include rigorous public exposure avoidance, early intervention to try and reduce disease severity, minimizing exposure to chronic B-cell depletion, and third dose vaccine strategies.
In Brief
These data provide limited information about the efficacy of adenovirus vaccines in these populations, and further studies are required to help guide vaccine choice in countries with access to both adenovirus and mRNA vaccines. Furthermore, although vaccine titers seem to correlate with protection against severe disease, more follow up is required to determine if this finding holds true for immunocompromised populations. Further studies in immunocompromised and high-risk cohorts, such as those with blood cancers, are urgently needed to guide vaccination and disease minimization strategies and to help identify patients who need rapid escalation of specific COVID-19 treatments.
Competing Interests
Dr. Michael Lane and Dr. Steven Lane indicated no relevant conflicts of interest.