Study Title: A Phase 3 Randomized Trial for Patients with De Novo AML Comparing Standard Therapy Including Gemtuzumab Ozogamicin (GO) to CPX-351 with GO, and the Addition of the FLT3 Inhibitor Gilteritinib for Patients with FLT3 Mutations (AAML1831)
ClinicalTrials.Gov Identifier: NCT04293562
Sponsor: Children's Oncology Group (COG)
Participating Centers: 177 institutions in the United States, Canada, Australia, and New Zealand
Accrual Goal: 1,400 children and adolescent/young adults younger than 22 years
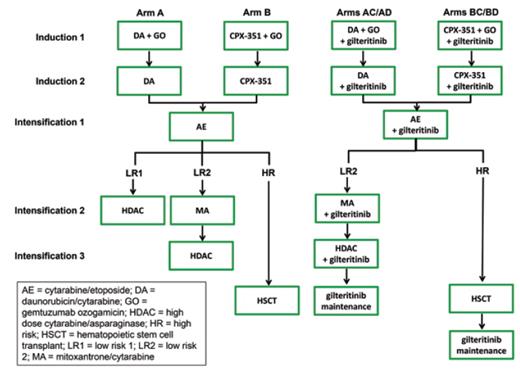
Study Design: AAML1831 is a randomized phase III clinical trial comparing the efficacy of standard multi-agent chemotherapy with gemtuzumab ozogamicin (arm A) to that of CPX-351, a liposomal formulation of cytarabine and daunomycin with a fixed 5:1 molar ratio, with gemtuzumab ozogamicin (arm B) and subsequent standard chemotherapy for children and adolescents/ young adults younger than 22 years who have newly diagnosed acute myeloid leukemia (AML; Figure). Patients are risk stratified as high-risk or low-risk based on AML-associated cytogenetic/molecular genetic alterations (Table) and end-induction measurable residual disease (MRD) assessed by centralized multi-dimensional flow cytometry. High-risk patients (identified by unfavorable genetics and/or MRD positivity ≥ 0.05%) are allocated to best-available donor allogeneic hematopoietic stem cell transplantation (HSCT) in first remission after three chemotherapy cycles, while low-risk patients are treated with chemotherapy alone. Low-risk patients with favorable AML-associated genetics and negative MRD receive four chemotherapy cycles (LR1), while low-risk patients with non-prognostic genetics and negative MRD receive five chemotherapy cycles (LR2) without HSCT. The FLT3 inhibitor (FLT3i) gilteritinib is added to standard chemotherapy or CPX-351/chemotherapy cycles for all patients with FLT3-internal tandem duplication (ITD) with an allelic ratio greater than 0.1 (arm C) or clinically-relevant FLT3-activating point mutations (arm D). FLT3-mutant patients also receive gilteritinib maintenance therapy for one year following HSCT or completion of chemotherapy (Figure).
Children's Oncology Group AAML1831 phase III clinical trial experimental design schema (NCT04293562). AE, cytarabine/etoposide; Allo, allogeneic; AR, allelic ratio; DA, daunomycin/cytarabine; DL, dose level; Gilt, gilteritinib; GO, gemtuzumab ozogamicin; HDAC, high-dose cytarabine/asparaginase (Capizzi II regimen); HSCT, hematopoietic stem cell transplantation; Ind, induction; Int, intensification; ITD, internal tandem duplication; MA, mitoxantrone/cytarabine; Maint, maintenance.
Children's Oncology Group AAML1831 phase III clinical trial experimental design schema (NCT04293562). AE, cytarabine/etoposide; Allo, allogeneic; AR, allelic ratio; DA, daunomycin/cytarabine; DL, dose level; Gilt, gilteritinib; GO, gemtuzumab ozogamicin; HDAC, high-dose cytarabine/asparaginase (Capizzi II regimen); HSCT, hematopoietic stem cell transplantation; Ind, induction; Int, intensification; ITD, internal tandem duplication; MA, mitoxantrone/cytarabine; Maint, maintenance.
Unfavorable (high-risk) and favorable (low-risk) childhood AML-associated genetic alterations used for risk stratification in AAML1831
| Unfavorable alterations | |
| Cytogenetics | Genetic fusion or involved genes |
| inv(3)(q21.3q26.2)/t(3;3)(q21.3q26.2) | RPN1-MECOM |
| t(3;21)(26.2;q22) | RUNX1-MECOM |
| t(3;5)(q25;q34) | NPM1-MLF1 |
| t(6;9)(p22.3;q34.1) | DEK-NUP214 |
| t(8;16)(p11.2;p13.3) if 90 days of age at diagnosis | KAT6A-CREBBP |
| t(16;21)(p11.2;q22.2) | FUS-ERG |
| inv(16)(p13.3q24.3) | CBFA2T3-GLIS2 |
| t(4;11)(q21;q23.3) | KMT2A-AFF1 |
| t(6;11)(q27;q23.3) | KMT2A-AFDN |
| t(10;11)(p12.3;q23.3) | KMT2A-MLLT10 |
| t(10;11)(p12.1;q23.3) | KMT2A-ABI1 |
| t(11;19)(q23.3;p13.3) | KMT2A-MLLT1 |
| 11p15 rearrangement | NUP98 fusion with any partner gene |
| 12p13.2 rearrangement | ETV6 fusion with any partner gene |
| Deletion 12p13.2 | Loss of ETV6 |
| Monosomy 7 | No associated gene |
| 10p12.3 rearrangement | MLLT10- any partner gene |
| No associated cytogenetic abnormality | FLT3-ITD with allelic ratio > 0.1% |
| RAM immunophenotype by flow cytometry | |
| Favorable alterations | |
| Cytogenetics | Genetic fusion or involved genes |
| t(8;21)(q21.3;q22) | RUNX1-RUNX1T1 |
| inv(16)/t(16;16)(p13.1q22.1) | CBFB-MYH11 |
| No associated cytogenetic abnormality | NPM1 mutation |
| No associated cytogenetic abnormality | CEBPA bZIP region mutation |
| Unfavorable alterations | |
| Cytogenetics | Genetic fusion or involved genes |
| inv(3)(q21.3q26.2)/t(3;3)(q21.3q26.2) | RPN1-MECOM |
| t(3;21)(26.2;q22) | RUNX1-MECOM |
| t(3;5)(q25;q34) | NPM1-MLF1 |
| t(6;9)(p22.3;q34.1) | DEK-NUP214 |
| t(8;16)(p11.2;p13.3) if 90 days of age at diagnosis | KAT6A-CREBBP |
| t(16;21)(p11.2;q22.2) | FUS-ERG |
| inv(16)(p13.3q24.3) | CBFA2T3-GLIS2 |
| t(4;11)(q21;q23.3) | KMT2A-AFF1 |
| t(6;11)(q27;q23.3) | KMT2A-AFDN |
| t(10;11)(p12.3;q23.3) | KMT2A-MLLT10 |
| t(10;11)(p12.1;q23.3) | KMT2A-ABI1 |
| t(11;19)(q23.3;p13.3) | KMT2A-MLLT1 |
| 11p15 rearrangement | NUP98 fusion with any partner gene |
| 12p13.2 rearrangement | ETV6 fusion with any partner gene |
| Deletion 12p13.2 | Loss of ETV6 |
| Monosomy 7 | No associated gene |
| 10p12.3 rearrangement | MLLT10- any partner gene |
| No associated cytogenetic abnormality | FLT3-ITD with allelic ratio > 0.1% |
| RAM immunophenotype by flow cytometry | |
| Favorable alterations | |
| Cytogenetics | Genetic fusion or involved genes |
| t(8;21)(q21.3;q22) | RUNX1-RUNX1T1 |
| inv(16)/t(16;16)(p13.1q22.1) | CBFB-MYH11 |
| No associated cytogenetic abnormality | NPM1 mutation |
| No associated cytogenetic abnormality | CEBPA bZIP region mutation |
Rationale: Liposomal anthracycline formulations have been reported as pharmacologically advantageous given prolonged time in circulation, altered biodistribution, and circumvention of drug efflux transporters,1 which may provide clinical benefit. A meta-analysis of nine randomized controlled trials of liposomal anthracyclines in adult patients demonstrated decreased cardiotoxicity, a major and potentially lifelong sequela of intensive AML anthracycline-based chemotherapy.2 The international Berlin-Frankfurt-Münster (iBFM) Study Group Relapsed AML 2001/01 phase III trial (NCT00186966) reported improved early treatment responses in children with first-relapse AML with fludarabine/cytarabine/granulocyte colony-stimulating factor (FLAG) reinduction chemotherapy and liposomal daunorubicin versus FLAG alone,3 though overall survival between the groups did not differ. The nonrandomized COG AAML1421 phase II trial also showed excellent clinical outcomes for children with first-relapsed AML reinduced with CPX-351 (cycle 1) and FLAG (cycle 2).4 A multi-center randomized phase III trial reported superior outcomes of CPX-351 versus conventional 7+3 cytarabine/daunomycin chemotherapy in older adults with newly diagnosed poor-risk AML.5 These studies provided strong rationale for the evaluation of CPX-351 in pediatric subjects with de novo AML.
Adding FLT3i to chemotherapy has improved outcomes in children and adults with FLT3-ITD AML,6–9 but studies of earlier-generation inhibitors (e.g., midostaurin, sorafenib) have also been complicated by toxicity due to multi-kinase inhibitory properties and/or development of resistance mutations. The next-generation selective FLT3i gilteritinib was chosen for pediatric investigation in AAML1831 based on clinical efficacy in adults with AML.10,11 In addition to children with FLT3-ITD AML for whom there are clear data to support FLT3i efficacy, those with FLT3-activating mutations are also treated with gilteritinib in AAML1831 based on potent preclinical activity and clinical safety data.12–14 However, it is plausible that resistance mutations will develop in gilteritinib-treated children over time, as has been reported in adult patients.15,16
Another significant feature of AAML1831 is its implementation of a revised molecular risk classification (Table). This updated classification based on next-generation sequencing analyses of banked COG and iBFM clinical trial specimens facilitated identification of new “boutique” high-risk genetic subsets of childhood AML with poor outcomes with chemotherapy alone.17–21
The predecessor COG AAML1031 trial showed that patients with genetically low-risk AML (Table) and negative end-induction MRD could be successfully treated with four instead of five chemotherapy cycles without increasing relapse risk.22 These favorable outcomes informed the empiric AAML1831 decision to exchange a traditional anthracycline-containing (mitoxantrone/ cytarabine) intensification 2 cycle for a high-dose cytarabine/asparaginase cycle with a goal of cardiotoxicity reduction for this population.
Comment: Survival of children with AML has plateaued despite maximally intensive therapy, and survivors remain at risk for lifelong treatment-related complications. AAML1831 aims both to improve survival and to reduce cardiotoxicity via investigation of liposomal cytarabine/daunomycin chemotherapy in the frontline setting, as well as to credential a selective FLT3 inhibitor in pediatric patients with FLT3-mutant AML. Importantly, this trial uses an evidence-based revised risk classification that will allocate a larger percentage of patients to HSCT in first remission due to high-risk genetics, while also reducing chemotherapy for children with favorable genetics and induction therapy responses to preserve good clinical outcomes while minimizing toxicities.
Competing Interests
Dr. Lamble and Dr. Tasian indicated no relevant conflicts of interest.