“Of all forms of inequality, injustice in health care is the most shocking and inhumane.”
–Martin Luther King Jr.
The recent report titled “Developing Healthy People 2020” defines a health disparity as “a particular type of health difference that is closely linked to social, economic, and/or environmental disadvantage.”1 Health disparities, therefore, disproportionately affect groups and populations that have long experienced higher barriers to optimal health based on their demographics, such as being African American or Black. In the past year, the COVID-19 pandemic has shed much light on the persistent and horrific health disparities experienced by African American and Hispanic populations, which are more likely to die from COVID-19 compared to white populations.2 Thirty-four percent of COVID deaths in the United States occurred among African Americans, who make up only 12 percent of the U.S. population.3
Sickle cell disease (SCD) predominantly affects individuals of African descent. In the United States, most people living with SCD are of African or Hispanic ethnicity; these groups are also more adversely affected by health disparities. The literature reports that individuals with SCD who progress to end-stage renal disease (ESRD) and who initiate dialysis have nearly twice the risk of mortality compared to those with comparable kidney disease but without SCD.4 While kidney transplantation is curative with the potential to significantly reduce this high renal disease–related mortality in SCD, available literature cites a two-fold higher mortality post renal transplantation in persons with SCD compared to those without SCD.5 These historical reports likely contribute to the resistance to referring individuals with SCD and ESRD to transplantation and may also contribute to the low conversion from waitlist to actual receipt of a kidney transplant.
A review of six observational studies of 311 individuals with SCD cited an 88 percent one-year post transplant overall survival.6 While outcomes and reporting differed among these studies, one report by Dr. Akinlolu O. Ojo and colleagues5 cited a 48 percent graft survival rate at three years versus 60 percent for patients without SCD (p=0.055) while another study a decade later reported graft survival rates at two, five, and 10 years that were comparable between individuals with and without SCD.7 The improved overall health status pretransplant and outcomes post-transplant for SCD over the past two decades may come as a result of the increased adoption of comprehensive care protocols, the use of hydroxyurea, and the overall improved supportive care available to persons living with SCD in the United States. This provides a more promising outcome for ESRD in SCD in current times, which shows a need for re-evaluation of the evidence supporting renal transplantation in SCD associated ESRD.
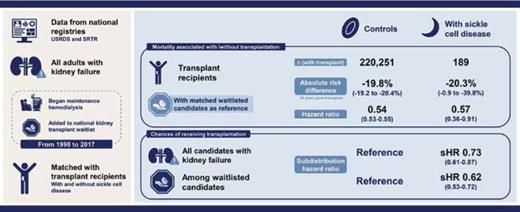
The manuscript by Dr. Sunjae Bae and colleagues sought to evaluate more contemporary data on the comparative risk of death from kidney failure and access to kidney transplantation among persons with and without SCD. It highlights how underappreciated the health disparities among SCD-associated ESRD in the United States chronic kidney disease population actually are. They queried national registries including the United States Renal Disease System and the Scientific Registry of Transplant Recipients that collects data on all adults with kidney failure, those who were started on dialysis, and those who either received or were waitlisted for renal transplantation between the decades spanning 1998 through 2017. Their main goal was to quantify the reduction in mortality from the institution of definitive therapy with kidney transplantation. They measured the absolute risk difference and hazard ratio (HR) of death between transplant recipients compared to matched waitlisted candidates with and without SCD. They also evaluated the chance of receiving a transplant for a patient with SCD compared to a matched control patient without SCD.
The SCD cohort in this analysis was almost two decades younger (median age interquartile range, 39 [31-49] vs. 53 [42-61] years), more likely to be African American (94% vs. 28%), less likely to have diabetes as a comorbid diagnosis (3% vs. 42%), and more likely to be publicly insured compared to their peers without SCD. Individuals with SCD however experienced a higher mortality across the three subgroups analyzed, namely dialysis, transplant waitlist, and post-transplant. At 10 years, the overall mortality estimates for dialysis, waitlisted, and post-transplant subjects was 85 percent, 61 percent, and 50 percent, respectively, for the SCD group compared to 81 percent, 41 percent, and 32 percent, respectively, in the non-SCD group. When adjusted for ethnicity, their model indicated higher adjusted HR of mortality of 2.14 (95% CI, 2.03-2.25) for dialysis mortality, 3.21 (95% CI, 2.84-3.62) for waitlist mortality, and 3.03 (95% CI, 2.42-3.80) for post-transplant mortality among individuals with SCD. These data suggest that proceeding to renal transplantation provided a survival advantage to all persons who develop ESRD, even those who were African American and those with SCD.
Kidney transplant was associated with the significant but quantitatively similar reduction in mortality across both the SCD and non-SCD groups at 20.3 percent and 19 percent, respectively. Individuals who received a kidney transplant experienced a 0.57-fold reduction in mortality in the SCD group compared to a 0.54 reduction in mortality among the non-SCD group. Unfortunately, despite being younger with fewer comorbidities and similar potential mortality benefit, only 38 percent of the waitlisted candidates with SCD ever received a transplant compared to 52 percent of waitlisted controls without SCD. Thus, individuals with SCD were less likely to receive a transplant (HR, 0.73; 95% CI, 0.61-0.87) or to be waitlisted for a transplant (HR, 0.62; 95% CI, 0.53-0.72), despite evidence to support its survival benefit for this subgroup. This clearly represents a hitherto unacknowledged but significant health disparity.
In 2019, ASH published evidence-based guidelines after a thorough review of the available literature on SCD and renal transplantation. They surmised in recommendation 7 that “renal transplant is justified [in SCD] given the high burden associated with dialysis and the comparable outcomes for patients with SCD versus diabetes and end-stage renal disease.”6 This conclusion was based on the moderate potential benefits of renal transplant in SCD with a trend toward better survival and reduced patient and caregiver burden with transplant compared to dialysis. Dr. Edmund Huang and colleagues reported improved survival in 173 individuals with SCD undergoing renal transplant compared with both the general population and persons undergoing renal transplant for diabetic nephropathy (SCD, 73.1%; diabetes, 74.1%; P = 0.44).8 Dr. Ojo and colleagues also reported a trend towards increased survival for 82 individuals with SCD following renal transplant compared to those who remained on dialysis (relative risk, 0.14; P = 0.056).5
There remains a dearth of high quality prospective comparative data on the post renal transplant outcomes in persons with SCD compared to those without SCD. Analyses using administrative datasets are retrospective, may not include individuals with SCD who were not waitlisted, may be region specific and non-representative of the entire SCD population, and may improperly assign subjects to the control group if the primary cause of their ESRD is not due to SCD. Nevertheless, the data clearly shows that renal transplantation was associated with substantial reductions in mortality similar between the SCD and non-SCD group, and yet access to transplantation remains a wide disparity in this population.
While not explicitly included in the kidney allocation criteria, the authors surmised that the higher post-transplant mortality for SCD transplants compared to non-SCD transplants poses an administrative disincentive to transplant centers who are judged on their aggregate one-year post-transplant mortality rates using diabetes-associated ESRD as the baseline metric. These metrics do not adjust for a recipient's diagnosis of SCD and potentially widens the disparity in access to transplant identified above.
In Brief
This study brings to mind the quote by Plato that states, “Nothing is as unequal as the equal treatment of unequals.” SCD-associated ESRD remains an unequal disproportionate health burden on those impacted. When treated as “equal” to diabetes-associated ESRD, the system negates the fact that a younger, healthier, ethnic minority population is dying two to three times more without the offer of definitive treatment with transplantation. Continuing this approach will widen the disparities in access to effective treatment and outcomes among the SCD population with profound loss of productive life years and increased health care costs.
Patients with sickle cell disease–associated kidney failure exhibited similarly lower mortality with kidney transplantation compared to those with other etiologies. Nonetheless, patients with sickle cell disease are less likely to receive transplantation, even after waitlist registration. Visual abstract by Michelle Lim, MBChB, MRCP; adapted with permission from Sunjae Bae et al. CJASN 2021;16:407-414.
Patients with sickle cell disease–associated kidney failure exhibited similarly lower mortality with kidney transplantation compared to those with other etiologies. Nonetheless, patients with sickle cell disease are less likely to receive transplantation, even after waitlist registration. Visual abstract by Michelle Lim, MBChB, MRCP; adapted with permission from Sunjae Bae et al. CJASN 2021;16:407-414.
Competing Interests
Dr. Osunkwo indicated no relevant conflicts of interest.