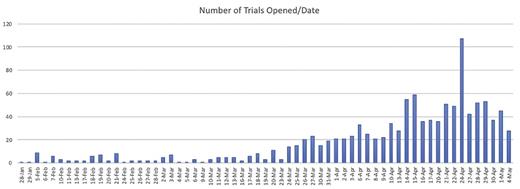
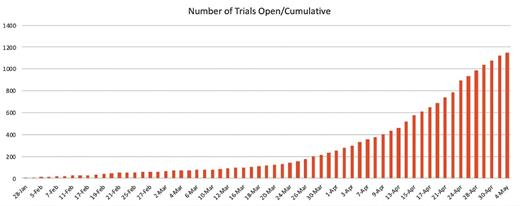
As part of ASH’s online coverage of the COVID-19 pandemic, we have been regularly cataloguing the number of trials being launched as recorded at ClinicalTrials.gov. The number of newly registered studies grew rapidly early in the pandemic — following a curve that mirrors that of the disease — exponential for much of February, March, April, and May of 2020 (Figure 1). More than two-thirds of the studies are evaluating efficacy of treatments or assessing general outcomes of patients with COVID-19 infection. The remaining studies are investigating tele-medicine, testing, mental health, and more (Figure 2).
It should be no surprise to readers that scientific discoveries from the labs of hematologists are finding their way into clinical trials for the novel coronavirus. Infection often results in a profound dysregulatory effect on blood function and production, which is why there has been so much controversy over anticoagulation strategies. It is also evident that the virus provokes, in some individuals, an unrestrained immune response. Hematologists are often called to treat conditions of immune dysregulation accompanied by organ dysfunction and profound immune response, including secondary hemophagocytic lymphohistiocytosis (sHLH) and the cytokine release syndrome (CRS) that can accompany chimeric antigen t-cell receptor therapy or the use of bispecific antineoplastic antibodies. In early May 2020, we culled all coronavirus studies listed on ClinicalTrials.gov; approximately 10 percent (108/1,160 studies) were investigating agents that are also used in hematologic disorders. Generally, most of these overlap agents are being tested owing to their known ability to blunt the immune response.
Data show elevated levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-6 (IL-6) in patients with severe COVID-19. For this reason, one of earliest categories of agents to be investigated are the IL-6 inhibitors.1 Tocilizumab is a humanized monoclonal antibody to the IL-6 receptor, which is familiar for its efficacy in the treatment of CRS. Parallel mechanisms have also led to investigations of sarilumab, clazakizumab, and anakinra. At the time this article was published, 41 different studies of IL-6 inhibition to manage coronavirus infection have been registered with ClinicalTrials.gov. The majority are testing these agents as monotherapy. Others are combining them with other immunosuppressive agents, antimicrobials, or in combination with complementary agents, like JAK inhibitors. No data has been formally reported from any of these trials to date. There are also three monotherapy studies looking at canakinumab, a human monoclonal antibody against interleukin-1β. Data support the use of this agent in, among other things, systemic juvenile idiopathic arthritis. The hope is that inhibition of interleukin-1β might downregulate inflammation.
Capitalizing on observations that GM-CSF is overexpressed, several inhibitors of GM-CSF are being studied in monotherapy trials. Lenzilumab, a human IgG1κ monoclonal antibody that has shown high affinity for human GM-CSF, has been tested in phase I studies for chronic myelomonocytic leukemia. It is one of three drugs in this category currently being studied for severe coronavirus-related pneumonia or acute respiratory distress syndrome.
A third category of agent familiar to hematologists, are inhibitors of the compliment cascade. Trials are being done on eculizumab or ravilzumab, both as monotherapy, given in vivo evidence that the complement system was an important host mediator of SARS-CoV–induced disease. A recently published letter describes,2 for example, four cases of patients with paroxysmal nocturnal hemoglobinuria and COVID-19, and the differential outcomes of those on complement inhibition versus those who were not — though no conclusions can be made from their observations.
Small molecular inhibitors make up the second largest class of overlap drugs for coronavirus. Ruxolitinib, an inhibitor of the JAK/STAT signaling pathway is commonly used for higher-risk myelofibrosis and has also shown benefit in graft-versus-host disease. We found 11 studies using this drug to treat the immune storm of coronavirus, including in combination with other agents. The first trial to publish results was a prospective, randomized study of 5 mg ruxolitinib orally twice-daily in patients meeting criteria for severe infection. A total of 43 patients were randomized to this placebo-controlled study which had criteria for clinical improvement by day 14 as a primary endpoint. The study did not show a statistical improvement in outcomes in the interventional arm, though the authors point out that three of 14 patients in the control group died of respiratory failure, while no patients died in the ruxolitinib group.3
Other small molecules being tested include agents to block Pi3K (duvelisib), Bruton tyrosine kinase (acalabrutinib), and even three studies testing imatinib. A 2016 publication on SARS-CoV and MERS-CoV documented that inhibitors of Abl-kinase, like imatinib, had in vitro activity against those coronavirus infections.4 Three studies are investigating inhibitors of the programmed-death pathway, employing nivolumab and pembroluzumab, along or in combination with IL-6 antagonists. Case reports on patients with receiving a programmed cell death protein-1 checkpoint inhibitor for cancer and contracting coronavirus have been mixed.
Finally, classical cytotoxic chemotherapy is also being investigated. Owing to its efficacy in the treatment of sHLH, one study is looking at etoposide as monotherapy. A second study will use methotrexate monotherapy in severe COVID infection due to its shared use in malignancy and autoimmune disease, presumed to assist in dampening the excess immune response contributing to lung injury in the “second phase” of COVID infection.
In summary, while we still have little definitive therapy that has been proven helpful in coronavirus, multiple innovative hematologic agents are being tested. We will see if the progress that we have seen in the past decade in managing blood cancers and high-risk disorders translates into control of this novel and devastating infection.
References
Competing Interests
Dr. Monahan and Dr. Michaelis indicated no relevant conflicts of interest.