Acute myeloid leukemia (AML) is primarily a disease of the older population, with a sharp spike in diagnoses seen in patients 60 years or older, and a median age of 70 years at disease onset. Current treatment outcomes for older patients with AML are limited by patient comorbidities, poor performance status, and altered drug metabolism. Additionally, disease-related factors include frequent adverse cytogenetic or molecular abnormalities. These factors contribute to low tolerability of treatments and overall reduced rates of clinical response to conventional treatments.1
B-cell lymphoma-2 (BCL-2) is an apoptosis inhibitory molecule that is overexpressed in most AMLs, allowing leukemia cells to put a brake on programmed cell death. Venetoclax is a specific BCL-2 inhibitor that can unleash apoptotic responses when the remaining components of this pathway, such as TP53, BAX, and PMAIP1, are functional.2-4 Clinical trials have revealed promising outcomes for AML patients. Although venetoclax as monotherapy showed only low response rates (19%) in relapsed/refractory AML, results dramatically improved when venetoclax was combined with low-dose cytarabine (LDAC; 54% complete response [CR]/CR with incomplete blood count recovery [CRi]; median overall survival, 10.1 months) or hypomethylating agents (DNMTi; 67% CR/CRi; median OS, 17.5 months) for older patients with de novo AML who were unfit for intensive chemotherapy.5,6 Unfortunately, relapse after venetoclax-based treatment remains common, and long-term cure remains elusive.
Dr. Courtney DiNardo and colleagues have investigated the genetic landscape underlying the clinical responses and resistance to venetoclax combination therapy for AML. Bulk AML as well as single-cell sequencing was performed on samples from 81 patients (median age 74 years) enrolled on two recently published trials of venetoclax in combination with either LDAC or DNMTi, taken at diagnosis, during remission, and at relapse.5 Survival was similar between the LDAC and DNMTi trials, and therefore, both cohorts were combined to increase statistical power. Patients were divided into three groups based on the quality of clinical response according to ELN2017 criteria: sustained remission (>12 months without relapse; n=18), initial remission with relapse (n=25), or primary refractory (n=20).
High rates of CR/CRi were found in patients with mutated NPM1 (93%) or IDH1/IDH2 (82-100%). The two-year survival outcomes (NPM1, 71.8%; IDH2, 79.5%) were particularly impressive and compare favorably with historical data for azacitidine (<25%) or intensive chemotherapy (<25%).6,7 Venetoclax treatment suppressed NPM1-mutated clones to undetectable levels in most cases, whereas IDH2-mutated clones remained present in about half of cases analyzed, suggesting that venetoclax may have specific activity dependent on the genetic driver of disease.
Conversely, resistance mutations after venetoclax combinations were identified in two main pathways. TP53 mutations were identified in eight (32%) of 25 relapsed AML cases and in seven (35%) of 20 primary refractory AML cases. Interestingly, the size of the TP53 clone increased in all serial samples analyzed, and the TP53 mutational burden at baseline was highest in the primary refractory group, whereas TP53 mutations were mostly undetectable at diagnosis in patients who developed late relapse. So, do these mutations occur de novo in the latter? It is more likely that TP53 mutations are already present in rare AML subclones, and this may be a concern in the older population. In fact, even an older person without a haematological malignancy will already harbor about 10 to 60 random mutations within the TP53 coding region by the age 70 years in their hematopoietic stem cell pool.8
FLT3-ITD and other kinase-activating mutations were identified in the adaptive resistance and primary refractory groups. Within the adaptive resistance group, these clones expanded at a rapid rate within one to six months from initial remission. Detailed single-cell sequencing revealed tremendous complexity of polyclonal resistance. In one example, FLT3-ITD, CBL, and NRAS mutations were initially detected, with outgrowth of five additional kinase mutations.
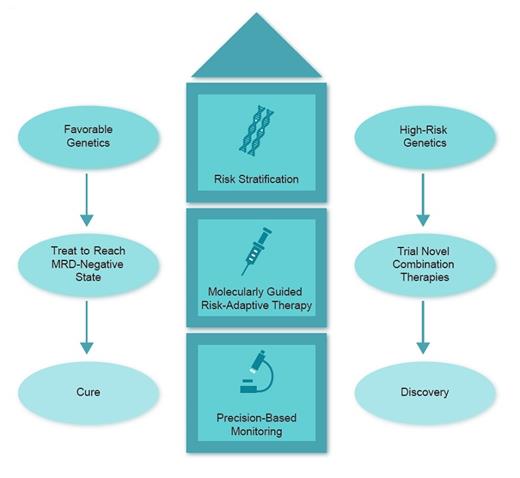
Schematic showing molecular stratification in acute myeloid leukemia
In Brief
Collectively, the results demonstrate the importance of molecular studies to identify patterns of drug resistance and response. Here, NPM1 and IDH mutations were associated with deep, durable responses. On the other hand, mutations in TP53 as well as FLT3-ITD and other kinase-activating mutations were correlated with relapse/refractory disease. It will be of interest to see how we can tackle the molecular complexity of AML through trials of new drugs and combinations, risk stratification, precision-based monitoring, and finally, molecularly guided risk-adaptive therapy (Figure). For example, future clinical trials may use molecular information to risk-stratify patients with AML by NPM1 or IDH mutational status as favorable with minimal residual disease (MRD) monitoring and MRD-directed outcomes. Combination trials with relapse prevention strategies such as sequential or combination FLT3 inhibitors, or agents with activity in TP53-mutant AML, are urgently needed.
References
Competing Interests
Dr. Bruedigam indicated no relevant conflicts of interest. Dr. Lane has participated in advisory boards for Novartis (midostaurin) and Astellas (gilteritinib).