Editor's Note
Normally in Clinical Trials Corner, I ask our Contributing Editors to summarize a single study. Here, Drs. Teachey and Si have reviewed three large studies that are moving immunotherapy into the treatment of children with acute lymphoblastic leukemia (ALL). While childhood ALL is often called out as the poster child for multiagent chemotherapy, there is still significant room for improvement in outcomes, and each of these trials has design elements that capitalize on what has been learned about prognostic markers at time of diagnosis and during the course of treatment.
Study Title:
A Study to Investigate Blinatumomab in Combination With Chemotherapy in Patients With Newly Diagnosed B-Lymphoblastic Leukemia (AALL1731)
ClinicalTrials.gov Identifier:
Participating Centers:
Children’s Oncology Group (COG) Institutions (anticipated to open at more than 220 centers in the United States, Canada, Australia, and New Zealand)
Accrual Goal:
6,720 patients
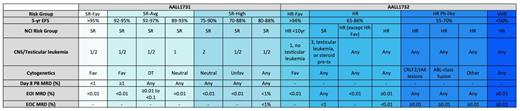
Adapted from a table provided by Rachel Rau, MD. Abbreviations: DT, double trisomies of 4 and 10; EOC, end of consolidation; EOI, end of induction; HR, high risk; HR-Fav, high risk favorable; MRD, minimal residual disease; PB, peripheral blood; SR, standard risk; SR-Avg, standard risk average; SR-Fav, standard risk favorable; VHR, very high risk.
Adapted from a table provided by Rachel Rau, MD. Abbreviations: DT, double trisomies of 4 and 10; EOC, end of consolidation; EOI, end of induction; HR, high risk; HR-Fav, high risk favorable; MRD, minimal residual disease; PB, peripheral blood; SR, standard risk; SR-Avg, standard risk average; SR-Fav, standard risk favorable; VHR, very high risk.
Study Design:
Currently, COG stratifies patients with B-cell acute lymphoblastic leukemia (B-ALL) into risk groups based on anticipated event-free survival (EFS); (Table 1). These risk groups are derived from parameters including age, white blood cell (WBC) count at diagnosis, presence or absence of extramedullary disease, disease response, and leukemic blast biology. AALL1731 is a phase III clinical trial that investigates the use of blinatumomab in combination with chemotherapy in patients with newly diagnosed standard risk (SR) or Down syndrome (DS) B-cell ALL. Blinatumomab is a bispecific single-chain antibody that targets CD19. Although the five-year overall survival (OS) rate for most patients with National Cancer Institute (NCI) SR B-ALL is greater than 90 percent, based on the relative number of these patients, they still account for approximately half of the overall relapsed population in childhood B-ALL.1 Patients with SR-average and SR-high disease (see Table 1 for definitions) will be randomized to standard chemotherapy or standard chemotherapy plus two cycles of blinatumomab. The rationale for use of blinatumomab in the SR population is based on the excellent outcomes seen in relapsed/refractory (r/r) B-ALL and the low toxicity profile.2 Data presented at the 2019 ASH Annual Meeting demonstrated that a subset of children with first-relapse B-ALL treated on the AALL1331 trial exhibited superiority and tolerability of blinatumomab compared to intensive chemotherapy prior to stem cell transplantation (SCT).3 The most common severe toxicities seen with blinatumomab are cytokine release syndrome (CRS) and neurotoxicity. Risk of CRS and neurotoxicity correlate with disease burden, and blinatumomab is only given to patients with low disease burden on AALL1731.2 The inclusion of patients with DS is based on inferior survival compared to non-DS patients from both increased treatment-related mortality and higher rate of relapse.4-6 Patients with DS and without consolidation failure, defined as end of consolidation (EOC) minimal residual disease (MRD) less than 1 percent, will receive three blocks of blinatumomab to replace selected cytotoxic chemotherapy to preserve antileukemia efficacy while reducing toxicity. Finally, AALL1731 will confirm if all patients can be treated with a uniform duration of therapy regardless of sex. This is the standard practice in many cooperative groups, whereas the COG has historically treated male patients with an extra year of therapy.
Study Title:
Inotuzumab Ozogamicin and Post-Induction Chemotherapy in Treating Patients With High-Risk B-ALL, Mixed Phenotype Acute Leukemia, and B-LLy (AALL1732)
Clinical Trials.gov Identifier:
Participating Centers:
COG Institutions (anticipated to open at more than 220 centers in the United States, Canada, Australia, and New Zealand)
Accrual Goal:
3,689 patients
Study Design:
CD22 is highly expressed in most cases of childhood B-ALL, making it an attractive target for therapeutic strategies.7,8 AALL1732 is a phase III randomized trial of inotuzumab ozogamicin (InO) for newly diagnosed patients with high-risk (HR) B-ALL. This trial will stratify NCI HR B-ALL patients into two risk groups (Table 1). Patients with NCI HR B-ALL who do not meet the HR-favorable definition and who have EOC MRD less than 0.01 percent will be randomized to receive or not receive two cycles of InO, an antibody drug conjugate composed of a humanized IgG monoclonal CD22-targeted antibody linked to calicheamicin. The trial’s primary endpoint is to determine if adding InO improves five-year disease-free survival (DFS). Patients with Ph-like ALL can remain on study or they can pursue alternative therapy but then would need to be removed from protocol therapy. Rationale for the use of InO in frontline therapy in patients with HR B-ALL is based on several adult clinical trials that have demonstrated impressive results in r/r B-ALL.9 AALL1621 is an ongoing phase II trial that prospectively evaluates InO’s toxicity profile and efficacy as a single-agent therapy in pediatric patients with r/r B-ALL. Data presented at the 2019 ASH Annual Meeting demonstrated complete remission (CR)/CR with incomplete hematologic recovery (Cri) rate of 58 percent, and 65.4 percent of responders achieved MRD less than 0.01 percent.10 More severe toxicities, including hepatotoxicity and sinusoidal obstructive syndrome (SOS), are seen with InO as compared with blinatumomab, which is part of the rationale to use this novel immunotherapy in a higher risk population with inferior EFS.9 AALL1732 will include nonrandomized interventions to investigate survival in B-ALL and mixed-phenotype acute leukemia when treated with standard therapy. Finally, AALL1732 will also confirm if all patients can be treated with a uniform duration of therapy regardless of sex.
Study Title:
Study of Efficacy and Safety of Tisagenlecleucel in HR B-ALL EOC MRD Positive Patients (AALL1712, CASSIOPEIA)
Clinical Trials.gov Identifier:
Participating Centers:
Anticipated to open in more than 50 centers in North America and Europe
Accrual Goal:
140 patients
Study Design:
CASSIOPEIA is a phase II, single-arm, open-label, multicenter trial whose purpose is to evaluate the efficacy and safety of tisagenlecleucel in pediatric and young adult patients (aged 1-25 years) with de novo HR B-ALL in first CR (CR1) who have EOC MRD 0.01 percent or higher. Tisagenlecleucel is a second-generation chimeric antigen receptor modified T cell, where autologous peripheral blood T cells have been genetically modified ex vivo to target CD19 on the surface of B-cells. The U.S. Food and Drug Administration (FDA) approval of tisagenlecleucel was based on results from the ELIANA trial that demonstrated OS of 90 percent at six months, and 79 percent at 12 months in children and young adults with r/r B-ALL.11 Investigators in the AALL1721 trial hypothesize that use of tisagenlecleucel not only offers the possibility of eliminating residual disease, but also could serve as definitive therapy without the need for consolidation with allogeneic SCT (aSCT). Patients who are not in remission are not eligible. Thus, tisagenlecleucel could also offer a better safety profile than seen in other trials; similar to blinatumomab high-tumor burden is associated with higher risk of severe CRS and neurotoxicity. Patients will be offered a second infusion of tisagenlecleucel if they have evidence of B-cell recovery less than six months after initial infusion or have re-emergence of MRD positivity without morphological relapse. Loss of B-cell aplasia less than six months after drug infusion has been associated with higher risk of relapse.12,13 The primary objective of this study is to compare the five-year DFS after tisagenlecleucel infusion with a historical control of chemotherapy and aSCT.
Commentary:
B-ALL is the most common childhood malignancy, and multiagent chemotherapy has led to an impressive cure rate higher than 85 percent.14 While stratifying patients and tailoring therapy based on risk of relapse has led to dramatic improvements in OS, many patients continue to relapse and have poor survival.1 Many of the improvements in survival within the past 50 years have been through intensification of cytotoxic chemotherapy regimens; however, we have reached a point where further intensification of conventional chemotherapies has proven too toxic, and any improvement in disease control is mitigated by treatment-related mortality. An attractive alternative mechanism to attempt to improve cures is the incorporation of immunotherapies. Multiple immunotherapies have demonstrated impressive efficacy in heavily pretreated populations and may offer the benefit of higher cure rates without excessive toxicity. The encouraging results in patients with r/r B-ALL serve as the basis for the investigation of three different immunotherapies in de novo patients with B-ALL. Future trials may take this approach further by eliminating more cytotoxic agents and combining immunotherapies in order to target multiple surface antigens and avoid relapse from antigen loss. There is still work to be done, as the development of effective immunotherapies that can be moved into the front line for T-cell ALL, infant ALL, and acute leukemia of ambiguous lineage are needed. Early-phase trials that include these populations are ongoing, and hopefully in the next few years we will see the paradigm shift for patients with these forms of leukemia.
References
Competing Interests
Dr. Si and Dr. Teachey indicated no relevant conflicts of interest.