Abbreviations: D, day; IV, intravenously; PO, by mouth; PS, ECOG performance score.
Abbreviations: D, day; IV, intravenously; PO, by mouth; PS, ECOG performance score.
In the past year, four highly important studies have been published each evaluating novel targeted agents for not-yet-treated chronic lymphocytic leukemia (CLL).1-4 Three of the trials tested ibrutinib-based combinations against standard immunochemotherapy. What made these trials important was the fact that ibrutinib went head-to-head against highly effective standards, rather the stacked-deck approach of comparing to an outdated regimen of chlorambucil monotherapy. The fourth trial evaluated a venetoclax-based combination. Let’s briefly review the four trials and synthesize what we have learned.
The ILLUMINATE trial randomized older patients with CLL to either ibrutinib-obinutuzumab or chlorambucil-obinutuzumab. The obinutuzumab and the chlorambucil were administered for six months while the ibrutinib was administered indefinitely. The likelihood of remaining in remission at 2.5 years was substantially higher in the ibrutinib arm (79% vs. 31%). The U.S. intergroup trial A041202 randomized older patients with CLL to either ibrutinib or ibrutinib-rituximab or bendamustine-rituximab (BR). BR was administered for six cycles while ibrutinib was administered indefinitely. The likelihood of remaining in remission at two years was substantially higher in either ibrutinib-containing arm (87% vs. 74%). The addition of rituximab did not enhance the efficacy of ibrutinib. The US intergroup trial EA1912 randomized younger CLL patients to either ibrutinib-rituximab or the fludarabine-cyclophosphamide-rituximab (FCR) regimen. FCR was administered for six cycles while ibrutinib was administered indefinitely. The likelihood of remaining in remission at three years was substantially higher in the ibrutinib-containing arm (89% vs. 73%).
Take homes? The Alliance trial teaches us that rituximab does not seem to improve the efficacy of ibrutinib. Whether this lack of benefit also holds true for obinutuzumab is unclear, as none of these trials addressed that question. However, a separate trial (ELEVATE) presented at the 2019 ASH Annual Meeting does suggest that obinutuzumab can increase the efficacy of Bruton tyrosine kinase inhibitors. Going forward, it seems reasonable to incorporate obinutuzumab with ibrutinib in the frontline setting.
Another teaching point from these three trials comes from a breakdown in outcome data by IgVH status. Immunochemotherapy typically performs far less well in IgVH unmutated CLL, and ibrutinib-based therapy is substantially more efficacious in this subgroup of patients. However, when analyzing the data in IgVH-mutated patients, the immunochemotherapy options perform very similarly to ibrutinib-based therapy, making these options worth discussing with IgVH-mutated patients.
A potential drawback to ibrutinib-based therapy is the need for continuous therapy. While ibrutinib is generally well tolerated, some patients experience vexing chronic toxicities, which can impair quality of life. Additionally, it is expensive, and the monthly copays can create financial strain. For these reasons, many research groups have been anxious to develop time-limited therapies using novel targeted agents, which brings us to the fourth trial. The CLL 14 trial conducted by the German CLL study group randomized older CLL patients to either venetoclax-obinutuzumab or chlorambucil-obinutuzumab. The obinutuzumab was administered for six months in each arm while the venetoclax and the chlorambucil were administered for 12 months. The likelihood of remaining in remission at two years was substantially higher in the venetoclax-obinutuzumab arm (88% vs. 64%).
So, when you see your next patient with CLL who is in need of frontline treatment, what should you offer? I find myself describing ibrutinib-obinutuzumab and venetoclax-obinutuzumab, attempting to address the pros and cons of each. It gets even more complicated for the IgVH-mutated patients, as I feel obligated to also describe an immunochemotherapy option. Often patients will declare their preference, and since none of these options have been compared head-to-head, I am happy to let patient preference break a tie.
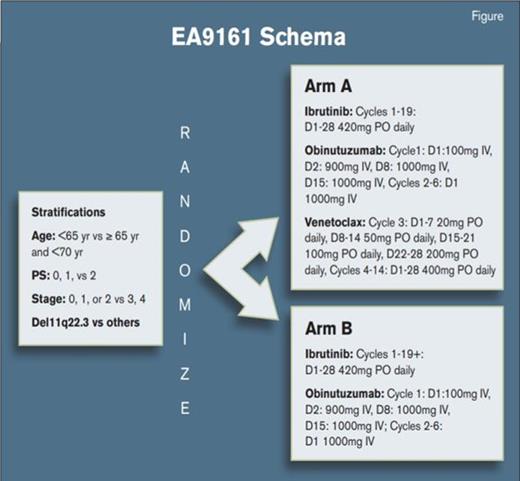
One way to simplify things is to participate in the two ongoing U.S. intergroup trials building off the success of the recent studies. EA9161 is designed for younger CLL patients (NCT03701282NCT03701282). The standard arm is ibrutinib-obinutuzumab given in the usual fashion. The experimental arm is a triplet of ibrutinib-obinutuzumab-venetoclax given in a time limited fashion. AO41702 is designed for older CLL patients and is similar in that it uses the same control arm (NCT03737981NCT03737981). The experimental arm uses the same triplet drug combination, but therapy discontinuation is based upon the results of planned minimal residual disease testing. Both trials are open and accruing briskly.
It is wonderful to see the U.S. cooperative group system working so well to both improve outcomes for CLL patients while establishing cost-effective and patient-friendly treatment paradigms.
References
Competing Interests
Dr. Kahl has served on advisory boards for Janssen, Pharmacyclics, Abbvie, Genentech, Acerta, and Astra Zeneca.