T-cell acute lymphoblastic leukemia (T-ALL) accounts for approximately 15 percent of pediatric, and 25 percent of adult ALL cases.1 The overall survival rate for pediatric patients currently exceeds 85 percent, but this requires intensive, prolonged therapy with short-term and long-term morbidity. Furthermore, patients with relapsed or refractory disease have a dismal prognosis.2 A powerful novel strategy for the treatment of patients with relapsed or refractory disease is immunotherapy. Immunotherapies have been applied to other types of ALL, specifically B-cell ALL (B-ALL), with great success; however, it has been challenging to apply this approach to T-ALL because of issues with fratricide and the inherent toxicities related to targeting T cells. In their recent article, Dr. Julie A. Hixon and colleagues describe a promising potential novel approach for the treatment of T-ALL via targeting the interleukin-7 (IL-7) receptor α (IL-7Rα). They developed a monoclonal antibody targeted against IL-7Rα and tested its safety and efficacy in preclinical models.
The investigators developed two murine monoclonal antibodies targeting IL-7Rα (constructs 2B8 and 4A10) and demonstrated their capacity to bind to mutant and wild-type IL-7Rα. Both antibodies were subsequently humanized. In an ex vivo experiment, both constructs effectively killed T-ALL blasts via antibody-dependent cell-mediated cytotoxicity (ADCC) using natural killer (NK) cells, with a higher effectiveness demonstrated when both constructs were combined. Fratricide of NK cells was not observed.
After demonstrating the effectiveness of both constructs in ex vivo experiments, the investigators used a series of patient-derived xenograft (PDX) models to further evaluate the efficacy and safety of this approach. They first demonstrated the efficacy of both constructs in an artificial leukemia driven by an IL-7Rα mutation that was introduced into immune-deficient mice. They then used a patient sample leukemia (T-ALL#5) to demonstrate the efficacy of the monoclonal antibodies in controlling low levels and high levels of disease and showed that the combination of 4A10 and 2B8 was effective in more samples than either antibody alone. Nevertheless, there was no difference in survival between single and combined antibody administration.
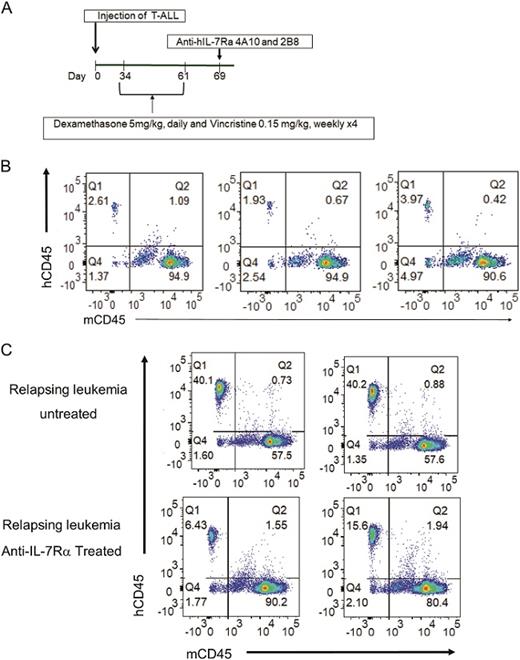
A. Experimental protocol. Patient derived T-ALL xenografts were established by intravenous injection into 20 NOD.SCID mice on day 0. When leukemia burden reached 1% in peripheral blood, mice were treated with a combination of dexamethasone (5 mg/kg, daily) and vincristine )0.15 mg/kg, weekly) for 4 weeks. On day 69, mice were given a single intravenous injection of anti-IL7Rα 4A10 and 2B8 (250 µg of each MAb) or PBS on day 69. B. Chemotherapy resistant leukemia was detected in the blood of mice at day 61 (n = 3). C. Growth of chemotherapy resistant leukemia was controlled by a single injection of anti-human IL-7Rα 4A10 combined with 2B8 at day 75 compared to mice that received chemo alone (n = 2).
A. Experimental protocol. Patient derived T-ALL xenografts were established by intravenous injection into 20 NOD.SCID mice on day 0. When leukemia burden reached 1% in peripheral blood, mice were treated with a combination of dexamethasone (5 mg/kg, daily) and vincristine )0.15 mg/kg, weekly) for 4 weeks. On day 69, mice were given a single intravenous injection of anti-IL7Rα 4A10 and 2B8 (250 µg of each MAb) or PBS on day 69. B. Chemotherapy resistant leukemia was detected in the blood of mice at day 61 (n = 3). C. Growth of chemotherapy resistant leukemia was controlled by a single injection of anti-human IL-7Rα 4A10 combined with 2B8 at day 75 compared to mice that received chemo alone (n = 2).
Finally, the authors established the efficacy of the anti-IL-7Rα in PDX models of relapsed T-ALL. PDX mice were treated with daily dexamethasone and weekly vincristine for four weeks, either alone or in combination. Following the completion of treatment, persistent or recurrent blasts were assessed for IL-7Rα expression using flow cytometry and were shown to have increased expression with both single agents and combination therapy. The increase in IL-7Rα was higher in patient samples treated with combination therapy as compared with those receiving dexamethasone alone. Results for mice receiving vincristine alone were not reported. Mice with relapsed leukemia following dexamethasone and vincristine were treated with either a combination of 4A10 and 2B8, or vehicle. Mice treated with the monoclonal antibody combination had reduced leukemia cells (measured by flow cytometry) 14 days post-treatment (Figure).
Treatment with the anti-IL7Rα monoclonal antibodies improved survival but was not curative. Despite exploring ex vivo testing in four unique samples of T-ALL, the PDX models used only a single sample (T-ALL#5). T-ALL samples are notoriously heterogeneous, and the efficacy demonstrated in T-ALL#5 has not yet been reproduced. The authors acknowledged this limitation, and further evaluation of T-ALL PDXs is underway. They also evaluated the efficacy of their antibody in relapsed T-ALL, using blasts from mice who had been treated with dexamethasone and vincristine in vivo to mimic, albeit imperfectly, relapsed T-ALL in patients. Perhaps the most important concern related to translating this discovery into the clinical realm is the broad expression of IL-7Rα on normal T-cells, as T-cell aplasia could be a major toxicity associated with this therapy.
In Brief
In summary, Dr. Hixon and colleagues developed two monoclonal antibodies targeting IL-7Rα and demonstrated their efficacy when used separately or in combination in ex vivo and in vivo models of T-ALL. Although potential toxicities need to be carefully considered, this study nevertheless represents an important potential breakthrough in the management of relapsed or refractory T-ALL.
References
Competing Interests
Dr. Diorio and Dr. Teachey indicated no relevant conflicts of interest.