Each year, approximately 20 percent of individuals taking a direct oral anticoagulant (DOAC) for stroke prevention will require an invasive procedure. Unfortunately, there is a wide discrepancy in opinion and scant published evidence to guide clinicians on the appropriate duration of treatment discontinuation. Patients and their physicians need to know when to hold an agent prior to procedures and how soon they should be restarted once treatment has concluded. This evidence gap puts patients at risk for thrombotic events as well as postprocedural bleeding.
Dr. James D. Douketis and colleagues performed a multicenter, prospective cohort study (PAUSE) that evaluated the safety of a standardized perioperative management strategy for patients with atrial fibrillation taking DOACs who required an elective surgery or procedure. Apixaban, dabigatran, or rivaroxaban were held for one to four days preoperatively based on the agent, the risk of procedure-related bleeding (high or low according to prespecified classification), and patient renal function. They were restarted two to three days postoperatively depending on the risk of postprocedural bleeding. Therapeutic-dose low-molecular-weight heparin (LMWH) was not permitted, and DOAC levels were not used to guide management. The primary outcome measure for each DOAC-specific cohort was major bleeding and arterial thromboembolism at 30 days.
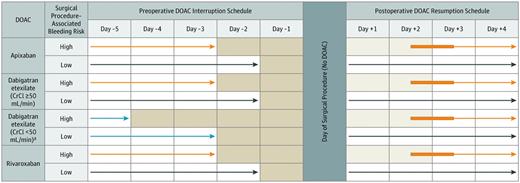
No DOAC was taken on certain days (shaded) and on the day of the elective surgery or procedure. The light blue arrows refer to an exception to the basic management, a subgroup of patients taking dabigatran with a creatinine clearance (C-CI) less than 50 ng/mi. The orange arrows refer to patients having a high-bleed-risk surgical procedure. Dark blue arrows refer to patients having a low-bleed-risk surgical procedure. The thickened orange part of the arrows refer to flexibility in the timing of DOAC resumption after a procedure. *Cancer diagnosed within 3 months or has been treated within 6 months or metastatic.
No DOAC was taken on certain days (shaded) and on the day of the elective surgery or procedure. The light blue arrows refer to an exception to the basic management, a subgroup of patients taking dabigatran with a creatinine clearance (C-CI) less than 50 ng/mi. The orange arrows refer to patients having a high-bleed-risk surgical procedure. Dark blue arrows refer to patients having a low-bleed-risk surgical procedure. The thickened orange part of the arrows refer to flexibility in the timing of DOAC resumption after a procedure. *Cancer diagnosed within 3 months or has been treated within 6 months or metastatic.
A total of 3,007 patients with atrial fibrillation (mean age, 72.5 years; mean CHADS2 score, 2) were enrolled (apixaban in 42%, dabigatran in 22%, and rivaroxaban in 36%). One-third of the procedures were classified as high bleeding risk. The 30-day postoperative rate of major bleeding was as follows: apixaban, 1.35 percent (95% CI, 0%-2.00%); dabigatran, 0.90 percent (95% CI, 0%-1.73%); and rivaroxaban, 1.85 percent (95% CI, 0%-2.65%). The 30-day postoperative rate of arterial thromboembolism was as follows: apixaban, 0.16 percent (95% CI, 0%-0.48%); dabigatran, 0.60 percent (95% CI, 0%-1.33%); and rivaroxaban, 0.37 percent (95% CI, 0%-0.82%). All major bleeding events and nine of 10 arterial events (ischemic strokes) occurred a median of two days (interquartile range, 0-6 days) postoperatively. Preoperative DOAC treatment levels were less than 50 ng/mL for more than 90 percent of the subgroup of 2,541 patients who had testing performed.
In Brief
This study confirms that a standardized perioperative management strategy for DOACs is safe, with low event rates for major bleeding and arterial thromboembolism. An additional noteworthy finding is that LMWH was not used for bridging. Switching patients from DOACs to LMWH to “bridge” around an invasive procedure does not make pharmacologic sense because the half-life of LMWH is similar to DOACs (8-14 hours). LMWH bridging for warfarin makes more sense owing to the much longer half-life of warfarin (40 hours). However, even this practice is questionable given recent data showing that it causes more harm than benefit for most patients.1
Does the PAUSE study answer all of our questions about perioperative management of DOACs? It does not, and in fact, it raises new ones. For example, rivaroxaban did not meet the prespecified threshold for major bleeding of less than 2 percent. Does this mean this agent should be held longer before and/or after procedures? Are these results applicable to dabigatran (with a smaller than planned sample size) or edoxaban (not included)? Is a DOAC-specific level of 50 ng/mL a safe cut point for high bleeding risk procedures, especially if neuroaxis anesthesia is required?
Another important observation from the PAUSE trial is the timing of the arterial thromboembolic events. The reported median was postoperative day 2, which is also when the risk of bleeding is still considered high for many procedures. A lower dose of DOAC given on postoperative day 2 might provoke less bleeding, but it is also less likely to be effective at preventing arterial thromboembolism. This suggests that at least some of these thrombotic events are not preventable.
The PAUSE study offers clear evidence-based guidance on how to manage DOACs around invasive procedures. It also provides a warning that clinicians and patients must agree to these procedures with their eyes wide open about the risks.
References
Competing Interests
Dr. Linkins was a data adjudicator for this trial, but was not involved in study design, data analysis, or writing of the manuscript.