Diagram detailing the structure and components of the ASH Research Collaborative Sickle Cell Disease Clinical Trials Network
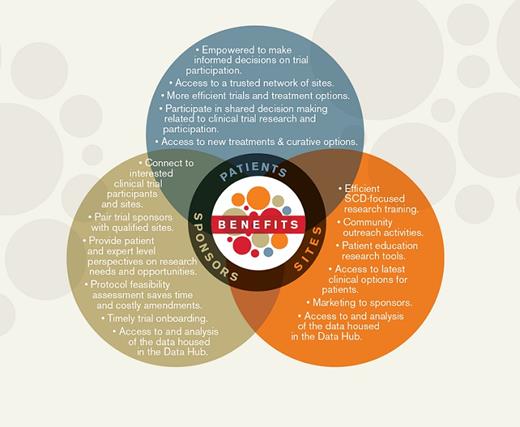
ASH Research Collaborative Sickle Cell Disease Clinical Trials Network Diagram.
ASH Research Collaborative Sickle Cell Disease Clinical Trials Network Diagram.
About a year ago, ASH publicly announced plans for a groundbreaking new effort — a Clinical Trials Network (CTN) for individuals living with sickle cell disease (SCD). Hours of debate and discussion preceded the decision and contributed to the development of a business plan. In the end, ASH leadership decided that this type of effort was exactly what the Society’s mission dictates: putting its resources behind a large, well-organized project that aims to accelerate the development of new therapies for patients with a devastating blood disease.
“ASH wanted to make a difference in a disease that probably hasn’t gotten a fair shake over a long period of time,” remembers Dr. Charles Abrams who serves as a chair of the ASH Research Collaborative SCD CTN Oversight Committee. “We held a conference with stakeholders to try and figure out how we could make the biggest difference in research, and overwhelmingly the CTN was supported as the most important action that ASH could take to make a difference in research.”
This comes at an opportune time. After years of therapeutic nihilism, there is growing evidence of novel ways to treat and manage SCD. In July 2017, the U.S. Food and Drug Administration (FDA) approved L-glutamine to reduce complications of vaso-occlusive disease. In the recent past, substantial progress in gene editing approaches has resulted in optimism for the potential of globin-mutation correction. The barriers to improving patient lives may no longer be a lack of ideas, but rather the logistical hurdles to proving these ideas effective and deploying them in the clinic.
“I think there are 43 different drugs at various stages in the pipeline. Some of them are curative, and some of them are small molecules that can probably be distributed to a much broader group of patients,” Dr. Abrams explained. “The landscape has certainly changed, and that has probably helped propel the CTN forward.”
Dr. Sophie Lanzkron, an SCD specialist at the Johns Hopkins School of Medicine and chair of the SCD CTN Patient Engagement and Education Subcommittee, says that she sees a great need for advocacy in the development of trials — not only to get patients involved in the design, but also to clarify to pharmaceutical companies exactly what is needed to ensure that these studies can enroll and reach the targeted population.
“There have been numerous trials that have closed because of low accrual, which is incredibly frustrating as someone who wants to do the best possible thing for my patients,” Dr. Lanzkron said.
But ASH has a great understanding of the challenges facing patients and trial development in SCD. “For me, being able to set up this network so that it will help with accrual is a key piece,” she continued, “but so is having this system where there is clear communication to industry about what the expectations should be to do these trials successfully.
“ASH fits that great niche between industry and the community of providers who care for individuals with SCD.”
In June 2018, ASH hired Dr. Charles Chesson to serve as director of the CTN and recruited Dr. LaTasha Lee to serve as Senior Manager of Partnership Engagement. Their first year of work has been to operationalize the endeavor, setting up the infrastructure and organizing both the patient engagement groups and nascent industry partnerships. Getting patients involved has been one of the important developmental milestones of the effort. So far, four of a planned eight patient engagement meetings have been undertaken. In these meetings, individuals diagnosed with SCD, and their caregivers and families discuss barriers to participation in trials and outline what they consider to be key research priorities. Following these meetings, Drs. Chesson and Lee aim to publish a comprehensive review of their findings, which will take the form of the first ever SCD Patient-Centered Research Agenda. The hope is that this agenda will influence research and be a driving factor in the development of additional treatment options for individuals living with SCD.
Simultaneous with patient engagement, the plan to approve the first wave of sites is on track and will be announced in the early fall or winter of 2019. Each of the sites is required to form a community advisory board as part of their application, ensuring that the views of parents, caregivers, teens, adolescents, and older adults with this disease are represented.
Dr. Abrams thinks that there is a growing momentum. “We’ve partnered with SCD community groups and patient national groups. We’ve met with the National Institutes of Health and with the FDA on several occasions. We have met with patient groups, and more recently, we’ve been engaging local sites that potentially would be members of the network.”
“There has been a lot of interest both by sites and by biotech and pharmaceutical companies,” he continued. “These milestones are getting buy-in from everyone. It’s been a joy to see that people are all stepping forward and saying, ‘We want to help with this.’”