X-linked severe combined immunodeficiency (X-SCID) is a rare inherited disorder of the immune system caused by mutations in the common γ chain (IL2RG). This condition is characterized by near-absent T and natural killer (NK) cells with present but dysfunctional B cells. Without immune reconstitution, X-SCID is generally fatal in the first year of life. The standard of care for X-SCID is allogeneic hematopoietic cell transplantation (HCT), with best outcomes achieved using HLA-matched sibling donors (MSD).1 However, less than 20 percent of infants will have an MSD, and transplantation with unrelated and haploidentical donors is complicated by increased rates of graft-versus-host disease (GVHD) and delayed immune reconstitution. Furthermore, high-dose chemotherapy is required for B-cell reconstitution in these patients, raising concerns for potential late effects related to the use of alkylating agents in early infancy.
Gene therapy is an appealing alternative for infants without an MSD because it allows for use of milder conditioning regimens and eliminates the risk of GVHD, abrogating the need for immunosuppressive therapy. Early gene therapy trials in Europe using traditional γ-retroviral vectors without conditioning demonstrated successful reconstitution of the T-cell compartment in patients with X-SCID.2-6 However, five of 20 patients developed T-cell leukemia due to insertional oncogenesis7-9 ; one patient died; and all patients remained dependent on immunoglobulin replacement. A subsequent international trial using a self-inactivating (SIN) γ-retroviral vector had an improved safety profile with no reports of myelodysplasia or leukemic transformation; however, humoral immunity was not restored in the absence of chemotherapy conditioning.10 More recently, Dr. Suk See De Ravin and colleagues at the National Institutes of Health (NIH) used a SIN-lentiviral vector with low-dose busulfan conditioning to treat five older male patients ranging from seven to 23 years of age with persistent immune dysfunction despite haploidentical HCT in infancy.11 Encouragingly, follow-up from the two older patients demonstrated both T- and B-cell reconstitution with clinical improvement and independence from immunoglobulin replacement.
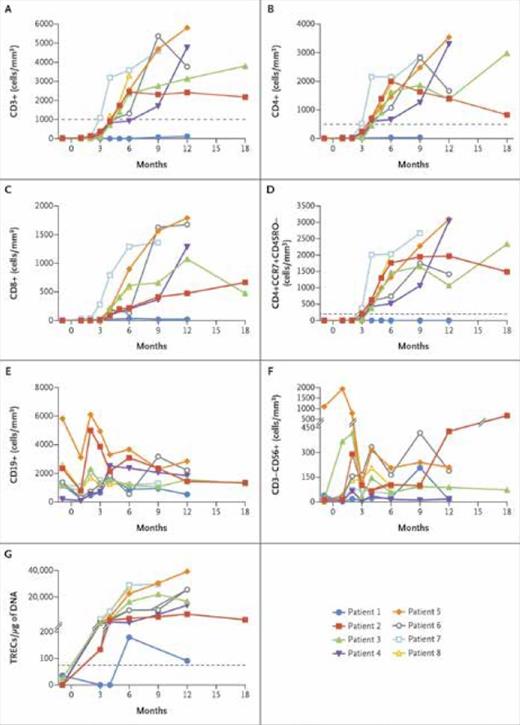
Dr. Ewelina Mamcarz and colleagues presented results of a dual-center, phase I-II clinical trial out of St. Jude Children’s Research Hospital and the University of California, San Francisco observing SIN-lentiviral gene therapy in eight consecutive male infants newly diagnosed with X-SCID and without an MSD. The group used the same lentiviral vector as the NIH to transduce bone marrow–derived CD34+ cells and low-dose targeted busulfan with a goal area under the curve (AUC) of 22 mg × h/L. Median age at gene therapy was 3.5 months (range, 2-14 months). Importantly, three patients had maternal T-cell engraftment, and five had a history of preexisting infection, including one patient with active cytomegalovirus (CMV) and two patients with active disseminated bacille Calmette-Guérin (BCG) at time of gene therapy. Median CD34+ cell dose was 6.73 × 106/kg (range, 4.46-15.10 × 106/kg), and median vector copy number (VCN) was 0.40 per CD34+ cell (range, 0.16-1.13 per CD34+ cell). Conditioning was well tolerated with no significant busulfan-related toxicity, and all patients were engrafted without the need for blood product transfusions. There were no significant new infectious complications, and the preexisting CMV and BCG infections were resolved with immune recovery. All patients were alive at a median follow-up of 16.4 months (range, 6.7-24.9 months). VCN has remained stable with time, and integration site analyses demonstrated highly polyclonal integration patterns in all patients.
Immune reconstitution after gene therapy. (A-F) Absolute numbers of peripheral-blood immune-cell subsets, as determined by means of standard flow cytometry, over time after cell infusion. Dotted lines indicate values for T-cell counts that are defined in the protocol as representing clinically significant reconstitution at 52 weeks after gene therapy. (G) Quantity of DNA T-cell–receptor excision circles (TRECs) in peripheral-blood mononuclear cells, with a dotted line indicating the lower limit of the normal range. The values above the hash marks on the y-axis range from 201 to 40,000. From The New England Journal of Medicine, Mamcarz E et al, Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1, Volume 380, Page 1531. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Immune reconstitution after gene therapy. (A-F) Absolute numbers of peripheral-blood immune-cell subsets, as determined by means of standard flow cytometry, over time after cell infusion. Dotted lines indicate values for T-cell counts that are defined in the protocol as representing clinically significant reconstitution at 52 weeks after gene therapy. (G) Quantity of DNA T-cell–receptor excision circles (TRECs) in peripheral-blood mononuclear cells, with a dotted line indicating the lower limit of the normal range. The values above the hash marks on the y-axis range from 201 to 40,000. From The New England Journal of Medicine, Mamcarz E et al, Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1, Volume 380, Page 1531. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
One patient had persistent maternal T cell engraftment and received a gene therapy boost 12 months after his first infusion with improvement in vector-marked T cells. Of note, this patient received the lowest CD34+ cell dose and had the lowest VCN of the graft. T-cell recovery was rapid in the remaining seven patients with all patients achieving normal T-cell subset counts (CD3+, CD4+, CD8+) within two to four months after infusion, including normal naïve T-cell counts (CD3+CCR7+CD45RO–; Figure). T cell receptor (TCR) excision circles were present in all seven patients at three months, indicative of thymopoiesis, and T-cell proliferation to phytohemagglutinin was normal by four months. Spectratyping demonstrated polyclonal TCR-Vβ repertoires in all patients. B-cell recovery was also rapid with normal B-cell counts by two months, and four patients were able to come off intravenous immunoglobulin replacement (IVIG) at 15 to 23 months after gene therapy. Three patients have also mounted protective antibody responses to vaccines, indicative of functional T- and B-cell reconstitution.
In Brief
In summary, early results from this phase I-II trial are encouraging, with 100 percent survival and seven of eight patients achieving rapid and robust T- and B-cell reconstitution following SIN-lentiviral gene therapy with low-dose busulfan. Immune reconstitution was more rapid than that typically seen following T-cell depleted unrelated and haploidentical donor HCT, and there were no cases of viral reactivation in this trial. Reassuringly, vector integration site analyses have demonstrated polyclonal integration patterns with no clonal expansions, though follow-up is admittedly short. Ultimately, long-term follow-up is needed to demonstrate durability of immune reconstitution and long-term safety of the lentiviral vector product. Nevertheless, results from this trial suggest that gene therapy is a viable treatment option for X-SCID patients without an MSD and may be superior to HCT with alternative donors. As more patients are treated with gene therapy and as safety and efficacy improve, one can foresee a time when gene therapy becomes the new standard of care for X-SCID.
References
Competing Interests
Dr. Arnold and Dr. Teachey indicated no relevant conflicts of interest.