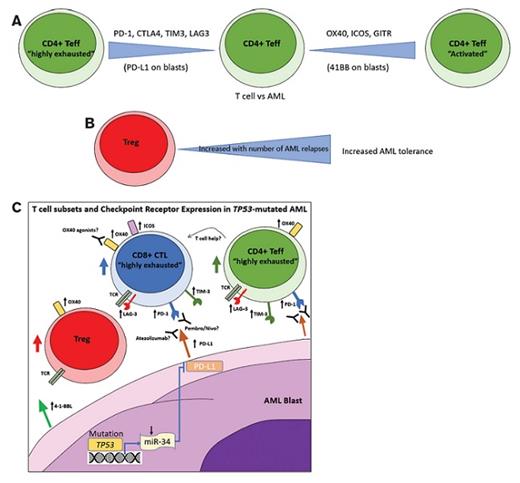
(A) Fine tuning of the T effector (Teff) response against AML though “activating” (OX40, ICOS, GITR, 4-1–BBL) and “exhausting” (PD-1/L1 CTLA4, TIM3, LAG3) checkpoint receptor-ligand pairs; (B) Increasing numbers of T regulatory cells (Tregs) with AML relapses leads to an increased immunosuppression and AML tolerance; (C) mutated TP53 leads to decreased transcription of microRNA-34 (miR-34) and subsequent transcriptional disinhibition of its target gene PD-L1 thus increasing expression of PD-L1 on AML blasts; increased numbers of Tregs and highly exhausted Teffs in AML impedes antitumor responses but may be amenable to PD-1/L1 axis antagonism by PD-1 inhibitors pembrolizumab/nivolumab or PD-L1 antagonist Atezolizumab; OX40 agonists may enhance tumor killing directly through CD8+ cytotoxic T cells (CTL) or indirectly through enhanced CD4+ Teff-mediated help.
(A) Fine tuning of the T effector (Teff) response against AML though “activating” (OX40, ICOS, GITR, 4-1–BBL) and “exhausting” (PD-1/L1 CTLA4, TIM3, LAG3) checkpoint receptor-ligand pairs; (B) Increasing numbers of T regulatory cells (Tregs) with AML relapses leads to an increased immunosuppression and AML tolerance; (C) mutated TP53 leads to decreased transcription of microRNA-34 (miR-34) and subsequent transcriptional disinhibition of its target gene PD-L1 thus increasing expression of PD-L1 on AML blasts; increased numbers of Tregs and highly exhausted Teffs in AML impedes antitumor responses but may be amenable to PD-1/L1 axis antagonism by PD-1 inhibitors pembrolizumab/nivolumab or PD-L1 antagonist Atezolizumab; OX40 agonists may enhance tumor killing directly through CD8+ cytotoxic T cells (CTL) or indirectly through enhanced CD4+ Teff-mediated help.
Checkpoint blockade therapy has revolutionized the therapeutic management of cancer and given rise to the burgeoning field of immuno-oncology. Checkpoint receptor–ligand pairs (e.g., programmed cell death protein 1 [PD-1] and its ligands [PD-L1/PD-L2]) play critical roles in the regulation of T-cell responses, particularly in the setting of exhaustion by chronic antigenic challenge, such as in cancer. Both activating (e.g., OX40, 4-1-BB, inducible T-cell costimulatory [ICOS]) and inhibitory (e.g., PD-1, cytotoxic T-lymphocyte antigen 4 [CTLA4], lymphocyte-activation gene 3 [LAG3], T-cell immunoglobulin, and mucin-domain–containing-2 [TIM3]) checkpoint molecules provide positive or negative signals that fine tune CD4+ and CD8+ T effector cells (Teffs; Figure 1A). T regulatory cells (Tregs), marked by co-expression of CD4, CD25, and FoxP3, maintain self-tolerance. Certain tumor types have co-opted these checkpoint mechanisms to evade antitumor T-cell responses. One of the best examples of this immune evasion strategy is through genetic alteration (i.e., polysomy, amplification, or rearrangement) of the 9p24.1 locus, which contains the genes encoding JAK2, PD-L1, and PD-L2, by classic Hodgkin lymphoma (cHL) and several subtypes of non-Hodgkin lymphoma.1
Checkpoint blockade strategies have enjoyed early clinical success in both hematolymphoid neoplasms and many solid tumors. Although objective response rates (ORR) vary widely based on tumor subtype (e.g., 15-25% in genitourinary, renal, and lung malignancies vs. 87% in cHL),2 many of these patient cohorts are in the relapse/refractory setting where therapeutic options are limited, emphasizing its clinical impact. Although much of the previous work has focused on tumors of lymphoid origin, within the past two to three years investigations into their role in myeloid neoplasms, particularly acute myeloid leukemia (AML), have begun. Robust immunophenotyping approaches, such as mass cytometry, have characterized the bulk cellular makeup of the tumor microenvironment in individual samples of AML but did not analyze T cells at the subset level.3 Others have investigated the functionality of T cells and expression of specific checkpoint markers in this setting, but also only in small patient cohorts.4 These early efforts have led to the initiation of several clinical trials set to evaluate checkpoint blockade therapy in AML, including in the post-transplant setting,5 in combination with hypomethylating agents in the relapse/refractory AML,6 and in a cohort of elderly AML patients,7 among others. Despite the initiation of these trials, a paucity of data exists in the evaluation of checkpoint markers and T-cell subsets in large cohorts of patients with AML.
Accordingly, Dr. Patrick Williams and colleagues of The University of Texas MD Anderson Cancer Center undertook a broad examination of T-cell frequencies, subset distribution, and checkpoint receptor expression patterns in 107 patients with AML (n=39 newly diagnosed, n=68 relapsed) and health donors (HD). Briefly, the study analyzed bone marrow and peripheral blood specimens by multiparameter flow cytometry for CD4+ and CD8+ Teff and Treg frequencies and expression of both activating and inhibitory checkpoint receptor and ligand expression on these T-cell subsets and AML blasts. Additionally, these data were correlated with conventional cytogenetic and mutational characteristics of the patients’ tumors.
Although the authors found no difference in absolute number or percentage of infiltrating CD3+ T cells between AML patients and HD, they found a significant enrichment of Tregs (p=0.02) in both newly diagnosed and relapsed AML patients versus HD with an increase in the number of Tregs with the number of relapses (Figure 1B). Additionally, Teffs are increased in AML compared to HDs with an upward trend with an increasing number of relapses, as measured by the frequency of PD-1+/CD4+ T-cells, OX40+/CD4+ T cells, and ICOS+/CD4+ T cells (p values: <0.01, <0.05, and 0.04, respectively). Importantly, the frequency of “highly exhausted” PD-1+/LAG3+/CD4+ or PD-1+/TIM3+/CD4+ Teffs, but not Tregs, tended to be increased in AML (p=0.05 and p=0.16, respectively) and particularly in the setting of relapse. The accumulation of these highly exhausted T cells has been associated with earlier relapse after allogeneic stem cell transplantation (ASCT) in AML patients8 and provides therapeutic rationale for anti–PD-1, anti-TIM3, and/or anti-LAG3 checkpoint inhibition in this setting.
The authors also sought to assess how particular genetic profiles of AML influence T cell subset distribution and checkpoint ligand expression on AML blasts. Although most parameters tested were not impacted by specific genetics, in patients with TP53-mutated AML, there was an increased frequency of PD-L1+ (p=0.05) and 4-1-BBL+ blasts (p=0.01), that is hypothesized to be mediated by the TP53/miR-34/PD-L1 pathway (Figure 1C). A similar trend was observed for PD-L1+ blasts in patients with adverse cytogenetics (p=0.09). They also observed increased Teffs but not Tregs in patients harboring mutations in DNA methylation pathways (DNMT3A, IDH1/2, TET2), which seemed to be independent of bone marrow blast burden. Taken together, these data suggest that AMLs harboring TP53 mutations or adverse cytogenetics may be more amenable to PD-1/PD-L1 axis inhibition, whereas those with DNA methylation pathway alteration may respond better to interventions that preferentially affect CD4+ Teffs.
In Brief
In summary, Dr. Williams and colleagues have examined T cell subset distribution and activating/inhibitory checkpoint receptor-ligand expression in the bone marrow microenvironment of AML using the largest cohort yet studied. The authors show increased frequencies of highly-exhausted Teff cells and non-exhausted Tregs in AML. These findings point to the mechanism of leukemic blast evasion from the immune system and provide a rationale for harnessing checkpoint-directed therapies as well as potentially other T cell approaches (e.g., bi-specific T-cell engager antibodies and chimeric antigen receptor T cells). Additionally, they found that TP53-mutated AML and AML with adverse cytogenetics have higher levels of PD-L1 and 4-1–BBL expression on their blast populations, implying that PD-1/PD-L1 axis antagonism may be of particular benefit in this cohort. Additional studies are required to assess checkpoint and T-cell subset temporal dynamics as well as expression on other key cell types of the microenvironment to complete the picture of leukemic immune evasion and fine tune these immune-directed therapeutic strategies.
References
Competing Interests
Dr. Wright and Dr. Kim indicated no relevant conflicts of interest.