The Case
A 55-year-old man presented with persistent shortness of breath and cough. A chest x-ray showed a mediastinal mass that was confirmed on computed tomography, along with extensive lymphadenopathy and splenomegaly. Although physical examination revealed no adenopathy or organomegaly, biopsy of the mediastinal mass demonstrated a diffuse large B-cell (DLBC) non-Hodgkin lymphoma (NHL). Complete blood count was normal as were liver function tests, except for moderate elevation in lactate dehydrogenase. The patient was started on rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). He was found to have severe neutropenia (absolute neutrophil count, 100 cells/µL) after one cycle of chemotherapy. Owing to the risk of future febrile neutropenia or sepsis, the treating hematologist recommended the addition of prophylactic granulocyte colony-stimulating factor with future cycles of chemotherapy. A new biosimilar pegfilgrastim was added to the formulary and was ordered with the next cycle of chemotherapy. The patient went on to tolerate the treatment regimen well with improvement in his symptoms of cough and mild peripheral neuropathy. Repeat imaging after two cycles of treatment revealed a greater than 50 percent reduction of the mediastinal mass and complete resolution of adenopathy and splenomegaly. Following his second treatment, the patient and his wife decided to continue treatment during the winter months at an institution in Florida. The institution has recently added biosimilar rituximab to the formulary.
The Question
Are there concerns about safety or efficacy of biosimilar pegfilgrastim for reducing the risk of febrile neutropenia in this setting? Furthermore, are there concerns about the safety and efficacy of biosimilar rituximab as part of the patient’s continuing treatment regimen for DLBC NHL?
The Response
Adapted with permission from Lyman G, et al. NEJM. 2018;378:2036-2044.
Adapted with permission from Lyman G, et al. NEJM. 2018;378:2036-2044.
Following an initial slow start, 18 biosimilars have been approved by the U.S. Food and Drug Administration (FDA) including 10 with immediate relevance to hematologists and oncologists. Approved biosimilars include supportive care agents represented by two filgrastim and two pegfilgrastim biosimilars, and more recently, cancer treatments including a biosimilar rituximab, with several more anticipated in the near future (Figure 1).1 While biosimilars have been on the market for more than a decade in Europe, the regulatory process, approval, and availability of these agents is a relatively recent addition to clinical practice in the United States. It is essential that providers familiarize themselves with the rationale for biosimilars; available evidence on safety and efficacy; terminology such as immunogenicity, drift, extrapolation, and interchangeability; as well as the novel drug nomenclature adopted to differentiate from the originator and other biosimilars. Several professional organizations and institutions have undertaken education initiatives to further inform providers, patients, administrators, and policy makers about biosimilars in hematology/oncology.2,3
The primary motivating driver of interest in the development and integration of biosimilars in clinical practice relates to the extraordinary increase in health care costs throughout the past decade. This is highlighted by rapid increases in cancer drug prices and most notably by the introduction of biologic therapies often associated with dramatic benefit, and simultaneously accompanied by high and escalating prices.4,5 While biologic therapies have unquestionably revolutionized treatment for cancer patients, this has been achieved at an extraordinarily high cost. The median price for newly approved biologic cancer therapies now well exceeds $10,000 per month, which is severalfold greater than the median monthly household income in the United States. Patients and their families are facing an ever-increasing financial burden potentially limiting access to optimal effective treatment with many health plans passing on a greater share of the cost of health care through coverage restrictions and high deductibles.
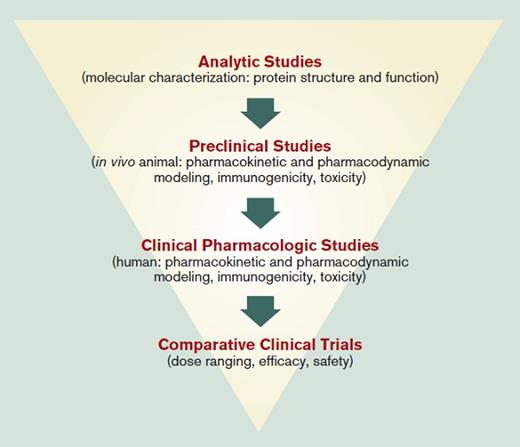
Biologic agents are large, complicated molecules produced in living organisms that cannot be fully characterized, may vary from lot to lot, and can induce antibodies that may block their drug efficacy. The FDA defines a biosimilar as “a biological product that is highly similar to a U.S.-licensed reference biological product for which there are no clinically meaningful differences in safety, purity, or potency of the product.”1 While differences will exist between the biosimilar and reference product, the amino acid sequence and the presumed mechanism of action will be the same. Although not identical to the reference product and requiring less clinical evidence than originators in order to reduce the cost of drug development, highly sophisticated molecular characterization, preclinical and human pharmacokinetic/pharmacodynamic testing, as well as immunogenicity testing are required (Figure 2). Additionally, there is considerable emphasis on postmarketing surveillance for any rare or delayed toxicities to assure high similarity with the reference agent.6 As with originator biologics, the molecular characteristics and behavior of biosimilars have the potential to drift over time, with relatively minor changes in manufacturing and ingredients requiring regular monitoring. Once approved for one indication, the FDA has the authority to grant approval based on extrapolation to other indications approved for the originator, without requiring additional clinical evidence for those indications. Although regulations granting a designation of interchangeability between biosimilar and originator have been put forward requiring additional costly clinical trials, no biosimilar company has yet sought or been granted such designation.
While biosimilars that support patients receiving cancer chemotherapy have been integrated into clinical guidelines and routine clinical practice, access to confirmatory research evidence by clinicians and professional organizations seems to be essential for guideline and practice integration.7,8 With careful regulatory evaluation, transparent reporting, and postapproval vigilance, the adoption and use of biosimilars will likely increase progressively in the United States, leading to greater price competition and improved patient access to these important and potentially curative agents. Evidence for considerable uptake and a modest but important influence on price associated with the introduction of the first filgrastim biosimilars into oncology practice has been reported.9,10 While patent challenges persist and no data are yet available, the greater convenience of pegfilgrastim for supporting patients receiving cancer chemotherapy would suggest that increasing use of these biosimilars is likely to follow suite.
Understandably, a greater challenge to biosimilar uptake is likely to be encountered with biosimilar forms of proven effective cancer therapies that have been available for decades and are often given with curative intent as in the use of rituximab in R-CHOP. The originator rituximab is a CD20-directed cytolytic antibody indicated for the treatment of NHL and several other malignant and nonmalignant diseases, originally approved by the FDA in 1997 with the patent ending in 2016 in the United States. While second-generation anti-CD monoclonal antibodies (obinutuzumab, ofatumumab) have been developed as alternatives to rituximab, several biosimilar versions of rituximab have been under development, and six have been approved in Europe. The first biosimilar rituximab was not approved in the United States until November 2018 for use in adults with CD20-positive, B-cell NHL as a single agent or in combination with chemotherapy. Of note, the phase III clinical data supporting the biosimilar rituximab applications have been conducted in patients with follicular NHL.11 Although biosimilar forms of rituximab have only been available in Europe since early 2017, early patterns of use have demonstrated early biosimilar rituximab use increased with later lines of therapy and is preferentially given to patients with indolent follicular lymphomas.12 Likewise, biosimilar rituximab was more likely to be used in patients with good performance status and few comorbidities. While recently under consideration, biosimilar rituximab is not currently recommended in National Comprehensive Cancer Network Guidelines for B-cell lymphomas. The acceptance and uptake of biosimilar rituximab in hematology/oncology practice for treating patients with NHL in the United States is yet to be seen.
Further studies and clinical experience, along with additional approvals leading to more competition will allow for further opportunities for appropriate integration of biosimilars of both the hematopoietic growth factors and targeted monoclonal antibodies into practice guidelines and clinical practice. Although it is too early to be certain, the resulting competition could have a meaningful effect on the unsustainable increase in the cost of care of patients with NHL and other hematologic and malignant disorders.
FDA-Approved Biosimilars
| 2015 . | 2016 . | 2017 . | 2018/2019 . |
|---|---|---|---|
| Filgrastim-sndz | Infliximab-dyyb | Infliximab-abda | Epoetin alfa-epbx |
| Etanercept-szzs | Adalimumab-adbm | Pegfilgrastim-jmdb | |
| Adalimumab-atto | Bevacizumab-awwb | Filgrastim-aafi | |
| Trastuzumab-dkst | Adalimumab-adaz | ||
| Infliximab-qbtx | Pegfilgrastim-cbqv | ||
| Trastuzumab-pkrb | |||
| Rituximab-abbs | |||
| Trastuzumab-dttb | |||
| Trastuzumab-qyyp |
| 2015 . | 2016 . | 2017 . | 2018/2019 . |
|---|---|---|---|
| Filgrastim-sndz | Infliximab-dyyb | Infliximab-abda | Epoetin alfa-epbx |
| Etanercept-szzs | Adalimumab-adbm | Pegfilgrastim-jmdb | |
| Adalimumab-atto | Bevacizumab-awwb | Filgrastim-aafi | |
| Trastuzumab-dkst | Adalimumab-adaz | ||
| Infliximab-qbtx | Pegfilgrastim-cbqv | ||
| Trastuzumab-pkrb | |||
| Rituximab-abbs | |||
| Trastuzumab-dttb | |||
| Trastuzumab-qyyp |
References
Competing Interests
Dr. Lyman has been a consultant to G1 Therapeutics, Halozyme Therapeutics, Partners Healthcare, Hexal, Bristol-Myers Squibb, Helsinn Therapeutics, Amgen, Pfizer, Agendia, and Genomic Health, Inc.