Study Title:
A Phase 2 Study of the JAK1/JAK2 Inhibitor Ruxolitinib with Chemotherapy in Children, Adolescents, and Young Adults with De Novo High-Risk Cytokine Receptor-Like Factor 2-Rearranged and/or JAK Pathway-Mutant Acute Lymphoblastic Leukemia
Clinicaltrials.gov Identifier:
Sponsor:
Incyte Corporation in collaboration with the Children’s Oncology Group (COG)
Participating Centers:
108 COG sites
Accrual Goal:
180 patients
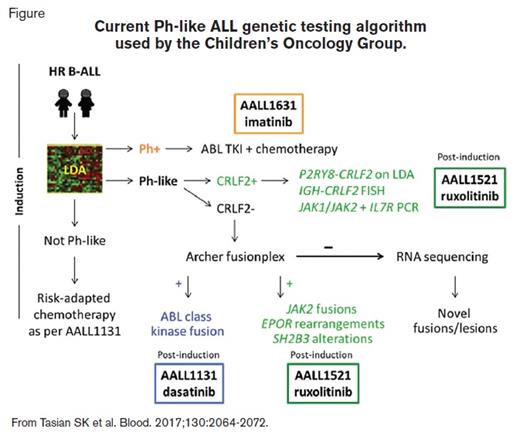
Schematic showing current Ph-like ALL genetic testing algorithm used by the Children's Oncology Group
Current Ph-like ALL genetic testing algorithm used by the Children’s Oncology Group.
Current Ph-like ALL genetic testing algorithm used by the Children’s Oncology Group.
Study Design:
AALL1521 is a nonrandomized, two-part phase II clinical trial designed to study the safety and efficacy of combining the JAK1/JAK2 inhibitor ruxolitinib with post-induction chemotherapy for pediatric patients with Philadelphia chromosome–like (Ph-like) B-cell acute lymphoblastic leukemia (B-ALL). This study is enrolling children, adolescents, and young adults ages one to 21 years with newly diagnosed National Cancer Institute (NCI) high-risk (HR) Ph-like B-ALL and CRLF2 rearrangements or other JAK pathway alterations following completion of a four-drug induction chemotherapy on or as per the COG AALL1131 phase III trial (NCT02883049).
Patients with HR B-ALL are screened for the Ph-like gene expression signature during induction via a low-density microarray (LDA) assay.1 Specimens identified as Ph-like undergo further genetic testing; those with CRLF2 overexpression are assessed for CRLF2 rearrangements (i.e., P2RY8-CRLF2 by reverse-transcriptase polymerase chain reaction [PCR], IGH-CRLF2 by fluorescence in-situ hybridization [FISH]) and for mutations in JAK1, JAK2, and IL7R by PCR, while those with normal CRLF2 expression undergo Archer fusionplex testing for ABL class, JAK2, EPOR, and other translocations. Rare Ph-like cases without identified lesions are allocated for RNA sequencing (RNA-seq) to identify novel mutations/fusions (Figure). Patients with the Ph-like signature and CRLF2 rearrangements, JAK2 or EPOR fusions, IL7R indels, or SH2B3 deletions are eligible for AALL1521 and are stratified into cohorts based on genetic lesion and end-induction minimal residual disease (MRD) status.
In the part 1 safety phase, investigators evaluated the safety of six dosing strategies in patients concomitantly receiving standard augmented Berlin-Frankfurt-Munster postinduction chemotherapy as per AALL1131. No dose-limiting toxicities were identified. The recommended phase II dose (RP2D) of ruxolitinib was determined to be 50 mg/m2/dose 14-days-on/14-days-off (dose level 2), and these interim results were presented at the 2018 ASH Annual Meeting.2 Part 2 of the trial is currently accruing to evaluate the efficacy of combination therapy. The primary endpoint of this trial is three-year event-free survival (EFS) of patients treated with ruxolitinib and chemotherapy versus 65 percent three-year EFS of historic control patients treated with an identical chemotherapy backbone. Secondary and exploratory outcomes include pharmacokinetic and pharmacodynamic assessments and rate of end-consolidation MRD negativity.
Rationale:
Despite high cure rates achievable in most children with B-ALL using risk-adapted chemotherapy, certain HR subsets have persistently very poor outcomes. Recent genomic profiling of patients with HR B-ALL led to identification of the Ph-like subtype,3,4 which comprises 15 to 20 percent of HR B-ALL in children and adolescents and nearly 30 percent in adults.5-9 Ph-like ALL is defined by the lack of BCR-ABL1 fusion but has a kinase-activated gene expression signature similar to that of Ph+ ALL, frequent deletions of IKZF1, and mutations in other kinase and cytokine receptor signaling pathway genes. Patients with Ph-like ALL have extremely high relapse rates and poor overall survival (OS). There are several main classes of genetic alterations described in Ph-like ALL to date: 1) ABL-class rearrangements involving ABL1, ABL2, CSF1R, and PDGFRB, and 2) JAK/STAT pathway alterations of CRLF2, JAK2, EPOR, IL7R, and SH2B3, as well as additional uncommon lesions in other kinase-associated genes.10 Approximately 50 percent of patients with Ph-like ALL have rearrangements in CRLF2 (often co-occurring with JAK2 or JAK1 point mutations) that result in constitutive JAK/STAT activation. An additional 8 to 16 percent of patients have JAK2 or EPOR translocations also characterized by hyperactive JAK/STAT pathway signaling. Preclinical studies have demonstrated in vitro and in vivo activity of ruxolitinib in Ph-like ALL models.11,12 The COG ADVL1011 phase I clinical trial (NCT01164163) previously demonstrated safety and tolerability of ruxolitinib monotherapy in children with relapsed/refractory cancers. These observations provide the rationale for frontline testing of JAK inhibitors in newly diagnosed patients with relevant genetic lesions and in combination with chemotherapy.13 Of note, patients with Ph-like B-ALL enrolled on AALL1131 with identified ABL-class fusions are nonrandomly allocated to receive dasatinib with post-induction chemotherapy.
Comment:
Addition of imatinib to chemotherapy for patients with BCR-ABL1-rearranged (Ph+) ALL dramatically improved EFS and revolutionized the paradigm for precision medicine in patients with acute leukemias.14,15 AALL1521 is a first-in-child investigation of the potential efficacy of combining JAK inhibition and chemotherapy to decrease relapse risk and improve OS in the relatively recently recognized Ph-like subtype of HR B-ALL. This trial is unique in its rigorous, real-time clinical characterization of Ph-like genetic lesions via gene expression analyses, FISH, PCR, unbiased fusionplex testing, and RNA-seq.
While a small number of anecdotal reports have described patients with Ph-like B-ALL and JAK pathway alterations who were treated with commercially available ruxolitinib and chemotherapy,16, the AALL1521 trial is systematically investigating key questions of whether the addition of a JAK inhibitor can decrease relapse and improve survival in patients with Ph-like B-ALL, as well as identify which genetic subgroups may or may not benefit from combination therapy. This trial represents a promising precision medicine strategy for targeting oncogenic drivers in a common and high-risk subset of B-ALL that spans the age spectrum.
References
Competing Interests
Dr. Ding and Dr. Teachey indicated no relevant conflicts of interest.