The advent of next generation sequencing (NGS) has revolutionized management of patients with myelodysplastic syndromes (MDS). From these analyses, TP53 mutant MDS, accounting for 5 to 10 percent of de novo MDS and 25 to 30 percent of therapy-related MDS, represent a specific cohort with the worst outcome (median overall survival [OS], 6-12 months).1 Specifically, these patients lack effective disease-modifying therapy compared to wild-type patients and display profoundly inferior OS with hypomethylating agent (HMA) therapy, which represents the standard of care in higher risk MDS.2-5 Furthermore, TP53 mutation is the only somatic gene mutation in all cohorts to predict inferior OS and minimal benefit from allogeneic hematopoietic stem cell transplantation.6-10 Additionally, we recently identified that TP53 variant allele frequency (VAF) is a key determinant of patient outcomes and further stratifies survival over binary mutation analysis and clinical prognostic models in MDS.4 Together, these studies highlight the profound negative connotation of TP53 mutation in MDS and the urgent need for effective, biologically rational, targeted therapies.
Reactivation of Mutant p53
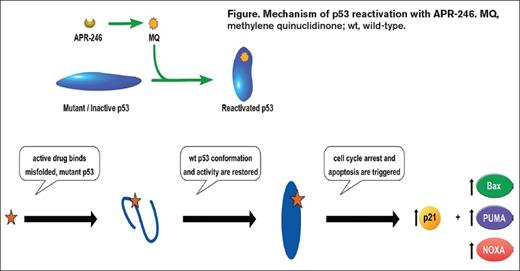
Clinical investigations in MDS are increasingly focused on exploiting biological consequences of specific somatic mutations as personalized therapeutic approaches, exemplified by IDH1/2 inhibitor and spliceosome inhibitor studies.11 But what about targeting TP53 mutation? One option recently studied in a prospective trial uses a novel small molecule anti-cancer compound, APR-246, that reactivates mutated and non-functional p53 and targets the cellular redox balance, two Achilles heels of cancer cells. This small molecule pro-drug is a methylated derivative and structural analogue of p53 re-activation and induction of massive apoptosis (PRIMA-1). Notably, PRIMA-1 was able to restore DNA binding and wild-type configuration and function in both contact and structural mutants.12 APR-246 spontaneously releases the active drug species, methylene quinuclidinone (MQ), at physiologic pH. MQ forms a covalent bond with cysteine residues in p53 — an event that thermodynamically stabilizes p53 protein, shifting the dynamic equilibrium away from the unfolded/misfolded state and toward the wild-type p53 conformation (Figure 1).13 Mutant p53 that achieves a wild-type conformation can then dimerize, and under conditions of cellular stress, form the tetrameric p53 species that drives transcription of targets that culminate in cell cycle arrest and apoptosis. A previous phase I study of APR-246 monotherapy that included patients with hematologic malignancy had on-target effects of p53 reactivation including induction of cell cycle arrest, apoptosis, and increased expression of p53 target genes (e.g., BAX, PUMA, and NOXA; Figure 1).14,15
In contrast to reactivating mutant p53, there has been substantial investigation evaluating activation of wild-type p53 via inhibition of the critical negative regulators of p53 (i.e., MDM2 and its homolog MDMX (also known as MDM4)).16 Amplification and/or post-translational modification of MDM2/MDMX lead to blockade of p53 transcriptional activity and consequent tumor formation and/or progression. RG7112 is a small molecule MDM2 antagonist with proof-of-concept phase I data showing objective responses and transcriptional activation of p53-target genes in AML patients.17 There are numerous additional small molecule inhibitors of MDM2 that have entered clinical study.18 Additionally, the stapled peptide ALRN-6924 is an antagonist of both MDM2 and MDMX and is being investigated as monotherapy and in combination with cytarabine in patients with MDS and AML. Importantly, MDM2/MDMX inhibitors may synergize with other novel therapies including agents that induce cell stress, BCL-2 inhibitors, and inhibitors of the BRAF/MEK pathway. Notably, the MDM2 antagonist idasanutlin is being combined with the MEK inhibitor cobimetinib or the BCL-2 inhibitor venetoclax in relapse/refractory AML, with clinical activity that has been recently reported.19 Because MDM2/MDMX inhibition requires functional p53, and one resistance mechanism is the development of TP53 mutant clones, novel combination of MDM2 antagonists and other p53 activating agents with mutant p53 reactivators would be of considerable interest in future studies.
Therapeutic Targeting of Mutant TP53 in MDS
Currently, the standard of care for higher-risk MDS is HMAs, though those with TP53 mutations rarely achieve durable clonal suppression and have poor OS.20,21 It’s this population that is being targeted in a phase Ib/II combination study of sequential APR-246 and azacitidine in HMA-naïve, TP53 mutant MDS and oligoblastic AML (≤ 30% blasts). The preliminary phase Ib results were presented at the 23rd European Hematology Association Congress in Stockholm, Sweden (Abstract S1558; ClinicalTrials.gov identifier NCT03072043). Five of six patients were response evaluable, with one patient discontinuing treatment prior to the first disease assessment. Overall response rate by the 2006 International Working Group Criteria was 100 percent, with 80 percent of patients (4 of 5) achieving complete remission (CR; 3 of 3 patients in dose level 2) and one marrow CR (mCR). Patients who achieved CR had deep molecular responses, with a median VAF at maximum clearance of 0.8 percent. As nuclear p53 positivity by immunohistochemistry is strongly concordant with TP53 mutational status and provides a functional readout of accumulated misfolded protein.,22,23 we are analyzing p53 IHC as a potential biomarker of response. Notably, all CR patients had high p53 positivity by IHC at baseline (55-70%), which normalized on serial assessment (< 5%). These data are encouraging and provide early proof-of-principle of clinical efficacy of p53 reactivation in the treatment of patients with MDS.
Conclusions and Unanswered Questions
As TP53 mutations represent the most common genetic alteration in cancer, occurring in approximately 50 percent of all invasive malignancies, developing targeted therapeutic strategies for mutant p53 proteins has far-reaching clinical applications. This is particularly relevant in hematologic malignancies in which TP53 has been identified as the most powerful negative prognostic covariate. Although mutant p53 historically has been considered an undruggable target, there is growing interest in compounds that reactivate mutant p53,24 with APR-246 being the most advanced in clinical development (Table). Defining the exact mechanisms of action of these agents with regard to restoration of wild-type function of p53 as well as impact on specific TP53 variants has yet to be resolved. Furthermore, understanding the downstream complex network of pathways that are impacted by transcriptional activation of p53 warrants investigation. It is notable that the clinical utility of these agents is likely in combination with other traditional and novel therapies, with unanswered questions focused on understanding how p53 reactivators can best synergize with these treatments. Personalized therapy in MDS has become possible through comprehensive understanding of the molecular architecture of an individual’s disease and optimally will translate into novel therapies for patients in the near future.
Novel Therapeutic Agents Targeting Mutant p53 Reactivation
| Class of Compounds . | Agent . | Novel Features . | Patient Population . | Stage of Development . | ClinicalTrials.govIdentifier . |
|---|---|---|---|---|---|
| Quinuclidines | APR-246 | Covalently bonds to mutant p53 → shifts equilibrium to wt confirmation; ↑ROS | HMA naïve HR-MDS; R/R melanoma, ovarian, and esophageal cancer | Phase 1-3 | NCT03072043; NCT02098343; NCT02999893; NCT03391050 |
| Pyrazoles | PK7088 | Reactivation of Y220C TP53 mutation | NA | NA | |
| 2-Sulfonylpyrimidines | PK11007 | ↑ thermal stability of mutant p53 to ↑ wt activity; ↑ ROS | NA | NA | |
| Zinc-metallochaperones | ZMC1 | Reactivates p53 mutant proteins with impaired zinc-binding; ↑ ROS | NA | NA | |
| Thiosemicarbazone | COTI-2 | ↑wt p53 confirmation; Inhibits PI3K/AKT/mTOR pathway | R/R Gynecologic Malignancies and Head and Neck SCC | Phase 1 | NCT02433626 |
| Small peptides | Peptide 46, CDB3, ReACp53, pCAP | Multiple mechanisms leading to ↑ wt properties | NA | NA |
| Class of Compounds . | Agent . | Novel Features . | Patient Population . | Stage of Development . | ClinicalTrials.govIdentifier . |
|---|---|---|---|---|---|
| Quinuclidines | APR-246 | Covalently bonds to mutant p53 → shifts equilibrium to wt confirmation; ↑ROS | HMA naïve HR-MDS; R/R melanoma, ovarian, and esophageal cancer | Phase 1-3 | NCT03072043; NCT02098343; NCT02999893; NCT03391050 |
| Pyrazoles | PK7088 | Reactivation of Y220C TP53 mutation | NA | NA | |
| 2-Sulfonylpyrimidines | PK11007 | ↑ thermal stability of mutant p53 to ↑ wt activity; ↑ ROS | NA | NA | |
| Zinc-metallochaperones | ZMC1 | Reactivates p53 mutant proteins with impaired zinc-binding; ↑ ROS | NA | NA | |
| Thiosemicarbazone | COTI-2 | ↑wt p53 confirmation; Inhibits PI3K/AKT/mTOR pathway | R/R Gynecologic Malignancies and Head and Neck SCC | Phase 1 | NCT02433626 |
| Small peptides | Peptide 46, CDB3, ReACp53, pCAP | Multiple mechanisms leading to ↑ wt properties | NA | NA |
Abbreviations: HMA, hypomethylating agent; HR-MDS, higher risk myelodysplastic syndrome; NA, not applicable; ROS, reactive oxygen species; R/R, relapsed/refractory; SCC, squamous cell carcinoma; wt, wild-type.
Acknowledgment: This work was supported by the MDS Foundation Young Investigator Grant.
References
Competing Interests
Dr. Sallman indicated no relevant conflicts of interest.