Study Title:
A Phase III Trial to Evaluate the Efficacy of the Addition of Inotuzumab Ozogamicin (a Conjugated Anti-CD22 Monoclonal Antibody) to Frontline Therapy in Young Adults (Ages 18-39 Years) With Newly Diagnosed Precursor B-Cell ALL
ISRCTN Number:
Sponsor:
Alliance for Clinical Trials in Oncology collaborating with National Cancer Institute
Accrual Goal:
310 participants
Participating Centers:
All Alliance sites (lead organization) and CTSU sites (including ECOG and SWOG).
Study Design:
This trial will enroll adults aged 18 to 39 years who have newly diagnosed, CD22+, Philadelphia chromosome–negative (Ph-neg) precursor B-cell acute lymphoblastic leukemia (B-ALL). The treatment backbone is the CALGB 10403 (C10403) pediatric-inspired ALL regimen — a complex, multiagent chemotherapy regimen administered in five parts: remission induction (course I), remission consolidation (course II), interim maintenance (course III), delayed intensification (course IV), and prolonged maintenance (course V). The study is designed to evaluate the benefit of adding two courses of inotuzumab ozogamicin (IO) at 0.5 mg/m2 on days 1, 8, and 15 in 28-day cycles between course I and course II.
IO has not been evaluated in combination with C10403. Therefore, the study will begin with an initial “confirmation of tolerability” phase in which the first six patients who respond to course I induction all receive IO before course II in a rolling six phase I design. Once a tolerable dose level is confirmed, the trial proceeds to full enrollment of the randomized phase III portion of the trial. In the phase III portion, randomization is stratified by presence or absence of Philadelphia-chromosome–like (Ph-like) signature, age (≤ 25 vs. > 25 years), CD20 status, and quality of response to course I (M0/M1 vs. M2).
The primary objectives of the study are to 1) confirm tolerability of adding IO to the C10403 regimen and 2) determine whether the addition of IO improves event-free survival (EFS) among patients who achieve response to course I of C10403. Secondary objectives include measuring other survival and response endpoints, characterization of toxicity and tolerability, and completion of correlative studies.
Rationale:
The retrospective recognition that young adults treated with pediatric regimens (which emphasize nonmyelosuppressive chemotherapy agents: asparaginase, steroids, and vincristine) fare better than those treated on adult regimens1 prompted multiple groups in the United States and Europe to prospectively test the safety and efficacy of applying pediatric-style regimens when treating adults up to 40 or 50 years of age with newly diagnosed ALL.2,3 The C10403 study applied the high-risk arm of the pediatric COG AALL0232 study to 317 patients aged 16 to 39 years and achieved a two-year EFS rate of 66 percent, representing a significant advance over historical outcomes with adult regimens that are associated with two-year EFS rates of 35 to 40 percent.3,4
Another major advance in the treatment of B-ALL has been the development of monoclonal antibodies including blinatumomab (a bi-specific T-cell engager of CD19 and CD3) and IO, a recombinant, humanized IgG4 antibody targeting CD22 conjugated with calicheamicin. CD22 is expressed in approximately 90 percent of cases of B-ALL and has advantageous pharmacologic properties including minimal shedding into the extracellular space and internalization after binding by IO, enabling selective targeting of CD22-expressing B cells by calicheamicin. Early-phase studies confirmed safety and efficacy of IO in patients with relapsed or refractory CD22+ B-ALL.5 Based on success of the subsequent phase III INO-VATE trial comparing IO with standard chemotherapy, IO was approved as a single-agent therapy for relapsed B-ALL.6
This study simultaneously applies two highly active therapeutic approaches — a pediatric-inspired chemotherapy regimen and the novel agent IO — to the initial treatment of adolescents and young adults (AYAs) diagnosed with Ph-neg CD22+ B-ALL. Relapsed ALL is a notoriously difficult entity to treat with few long-term survivors, even among those who initially respond to novel salvage approaches.6-8 By combining multiple successful approaches in the upfront treatment of B-ALL, this trial represents an effort to continue to reduce the number of patients who relapse.
One concern about adding IO to the C01403 regimen is the potential for hepatic toxicity in the form of sinusoidal obstruction syndrome (SOS), which was reported in the phase III INO-VATE study.9 This toxicity was primarily seen in patients proceeding to stem cell transplantation (SCT) with double-alkylating agent conditioning. To minimize the risk of SOS in this trial, the dose of IO in each cycle is lower than in INO-VATE (1.5 mg/m2 vs. 1.8 mg/m2), and the number of cycles is capped at two. Additionally, based on an already low rate of SCT in C10403, it is anticipated that the need for SCT will be infrequent in this study. For these reasons the investigators expect a low rate of SOS in the IO treatment arm; however, patients will be observed closely for hepatic toxicity.
Comment:
While the clinical efficacy of combining an AYA chemotherapy regimen with a novel antibody is the key question being addressed by this trial, large cooperative group trials of rare diseases offer precious opportunity to study even rarer subgroups. This fact is not lost on this study’s investigators, who carefully designed correlative sub-studies to accompany the clinical trial.
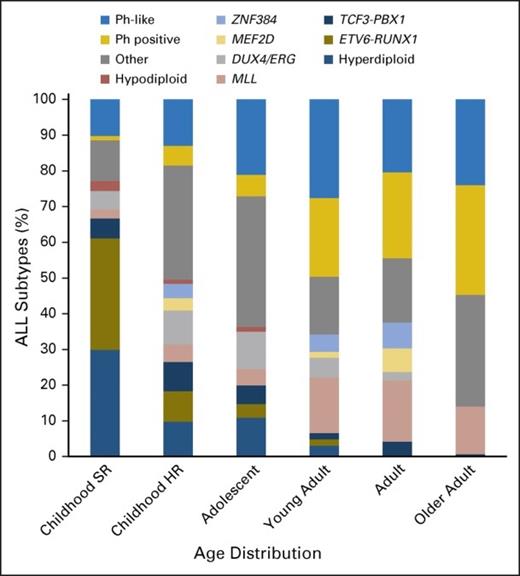
Recent research has not only led to advances in therapeutics for B-ALL, but also revealed new details about the genetic landscape of the disease. Ph-like ALL is a subgroup of B-ALL defined by a gene-expression profile that mimics that of Philadelphia chromosome–positive (Ph-pos) ALL.10,11 Ph-like ALL is characterized by a wide range of genomic alterations that result in kinase-dependent pathway activation.10,12 Although initially described as a high-risk subgroup in the pediatric ALL population, Ph-like ALL is actually most prevalent in AYAs (age 16-39 years), where 19 to 28 percent of patients fall into this subtype (Figure).11-13 This trial will allow in-depth genetic characterization of Ph-like ALL as well as permit prospective assessment of the impact of Ph-like status on minimal residual disease (MRD) and clinical outcomes, as well as assess the effects of IO based on Ph-like status.
The prevalence of ALL subtypes varies in children with standard-risk (SR) ALL (age 1 to 9 years and WBC count < 50 × 109/L), children with high-risk (HR) ALL (age 10 to 15 years and/or WBC count > 50 × 109/L), and adolescents (age 16 to 20 years), young adults (age 21 to 39 years), adults (age 40 to 59 years), and older adults (age 60 to 86 years) with ALL. Other, B-cell ALL lacking recurrent abnormalities; Ph, Philadelphia chromosome. Data adapted. Reprinted from Iacobucci and Mullighan with permission.
The prevalence of ALL subtypes varies in children with standard-risk (SR) ALL (age 1 to 9 years and WBC count < 50 × 109/L), children with high-risk (HR) ALL (age 10 to 15 years and/or WBC count > 50 × 109/L), and adolescents (age 16 to 20 years), young adults (age 21 to 39 years), adults (age 40 to 59 years), and older adults (age 60 to 86 years) with ALL. Other, B-cell ALL lacking recurrent abnormalities; Ph, Philadelphia chromosome. Data adapted. Reprinted from Iacobucci and Mullighan with permission.
Unlike in Ph-pos ALL, Ph-like ALL is not defined by a single genetic lesion. Instead it is a group composed of a panoply of alternate fusions and mutations in kinase genes that makes identifying this subgroup challenging.10,14 Although initial categorization of the Ph-like subtype used gene expression arrays, a minimum set of eight mRNAs has been optimized that can be assessed by quantitative real time polymerase chain reaction (RT-PCR) on a multianalyte card — the Taqman low-density array (LDA) card — with a single integrated expression score.14 This study will assess Ph-like status using the LDA card and also conduct in-depth genomic analyses to identify the specific gene fusions and mutations associated with the Ph-like signature. Patient responses in the two arms of the trial will be correlated with Ph-like signature status, and there is the potential to describe outcomes based upon specific fusion types, although the trial is not powered specifically for such analyses. Interestingly, Ph-like status via LDA card will be assessed at diagnosis and used for randomization, but it will only be shared with the investigator if the patient relapses. Although some investigators will know the Ph-like status of their patients based on local assessments, no established therapeutic interventions for this subgroup exist; hopefully any local knowledge will not significantly affect the ability to interpret the results of this trial.
Another important aspect of this study is rigorous, centrally determined MRD assessment at prespecified time points. While MRD status is well-established as a prognostic biomarker in B-ALL, not all MRD assays are the same, with a variety of established and emerging methodologies being worthy of further study. Most widely used are multiparametric flow cytometry MRD assays, which can identify a leukemia-associated immunophenotype in more than 90 percent of patients and detect one in 10,000 leukemic cells (analytical sensitivity of 10-4).15 A less widely used and more laborious method for assessing MRD is allele-specific oligonucleotide RT-PCR (ASO-PCR), which uses PCR primers designed for a patient's individual immunoglobulin sequence. Using the ASO-PCR method, more than 95 percent of B-ALL patients will have a trackable rearrangement with an analytical sensitivity of 10-4 or 10-5.15 More recently, next-generation sequencing methods have been shown to identify clonal rearrangements in all patients and achieve remarkable analytical sensitivities of 10-6.16 This latter method can also identify and monitor multiple clonal population and characterize clonal evolution. This IO study will measure MRD using multiple methods (flow cytometry, ASO-PCR, and possibly next-generation sequencing) to learn the prognostic implications of MRD quantification by various methodologies and to provide further understanding of the effect of Ph-like status and IO on MRD response.
Recently, this study has cleared the initial tolerability phase and is accruing to the randomized phase III portion. This trial represents a promising approach to improving outcomes for AYAs, as well as an unparalleled opportunity for scientists to better define ALL disease biology, which will hopefully lead to further treatment advances.
References
Competing Interests
Drs. Luskin, DeAngelo, and Kim indicated no relevant conflicts of interest.