Historically, for patients with acute lymphoblastic leukemia (ALL), remission has been defined by morphology (< 5% lymphoblasts in bone marrow with hematologic count recovery). Remission status after initiation of therapy has critical implications because it can affect eligibility for salvage regimens or experimental agents on clinical trials. Failure to obtain remission after the first block of chemotherapy is sometimes used to determine if a patient should undergo hematopoietic stem cell transplantation (HSCT). Morphologic complete remission (CR) rates are often used for regulatory approval of novel therapies, for determination of success or failure of new agents in early-phase trials, and for comparison of the activity of new agents or blocks of therapy across trials. Ironically however, morphologic assessment of remission is not always straightforward because lymphoblasts are histologically very similar to hematogones (normal B cell precursors), and manual enumeration can be inaccurate when blasts are not evenly distributed in the bone marrow.
Measurement of minimal residual disease (MRD) after initiation of therapy has been shown in multiple studies to be the strongest predictor of outcomes for children and adults with ALL.1 MRD is used for risk stratification by all major cooperative groups because intensification of therapy for patients with poor MRD response often improves outcomes. MRD can be measured by multiple techniques, including flow cytometry, polymerase chain reaction (PCR), and next-generation sequencing. These techniques are more robust than morphology and overcome many of its limitations. Despite the power of MRD, most cooperative groups continue to rely on morphology to define remission.
The Medical Research Council (MRC) recently reported inferior outcomes in patients treated on the UKALL 2003 trial who had morphologic remission at the end of induction therapy and MRD of 5 percent or greater (“high MRD”) using PCR-based MRD.2 Based on these results, they proposed a new definition of induction failure (i.e., failure to obtain remission) as 5 percent or greater residual blasts either by morphology or by MRD. The authors emphasized that these results need to be verified in additional trials and with different MRD technologies.
Dr. Sumit Gupta and colleagues recently published the outcomes of children with discordant measurements of remission by MRD and morphology.3 They compared outcomes in 9,350 children and young adults with de novo B-precursor ALL (B-ALL) and T-precursor ALL (T-ALL) treated on Children’s Oncology Group (COG) trials. On these trials, morphology was performed locally, and MRD was performed centrally in one of two laboratories. By morphology, patients were clustered into three groups: M1 (<5% lymphoblasts), M2 (5%-25% lymphoblasts), or M3 (>25% lymphoblasts). Most patients were in a morphologic remission at the end of induction (M1: 9,148 patients [97.8%]; M2: 118 patients [1.3%]; M3: 84 patients [0.9%]), and overall, morphology and MRD were concordant in most patients (9,111 patients; 97.4%).
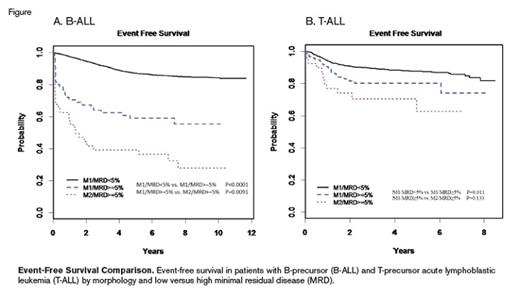
Event-free survival in patients with B-precursor (B-ALL) and T-precursor acute lymphoblastic leukemia (T-ALL) by morphology and low versus high minimal residual disease (MRD).
Event-free survival in patients with B-precursor (B-ALL) and T-precursor acute lymphoblastic leukemia (T-ALL) by morphology and low versus high minimal residual disease (MRD).
Discordance happened in both directions in that 40 patients had M2/M3 morphology and MRD less than 5 percent (“low MRD”; 19.8% of M2/M3 patients had discordant MRD), and 164 patients had M1 morphology and greater than 5 percent blasts by MRD. Discordance was more common in patients with T-ALL (97 [6.9%] of 1,493 patients) than those with B-ALL (66 [0.9%] of 7,857 patients). B-ALL patients with M2/M3 morphology and low MRD had inferior survival (5-year overall survival [OS]) compared with patients who were concordantly in remission (Table; Figure). Nevertheless, 17 (85%) of 20 patients with MRD less than 1 percent and M2/M3 were alive at last follow-up, compared with four (40%) of 10 M2/M3 patients with low MRD that was greater than 1 percent, suggesting there may be an MRD cutoff that can identify M2/M3 patients with favorable outcomes. A pooled analysis including patients from other cooperative groups may provide sufficient power to make that determination. For patients with T-ALL, the five-year OS was no different comparing M2/M3 morphology and low MRD with those who were concordantly in remission (Table), but only 10 patients had M2/M3 and low MRD.
OS and EFS Comparison of Discordant High-Morphology and Low-MRD Versus Concordant Morphology/MRD
| OS | B-ALL | M2/M3 and MRD <5% | 72.7 +/– 9.8% | M1 and MRD <5% | 93.8 +/– 0.3% | p<0.0001 |
| T-ALL | M2/M3 and MRD <5% | 100 | M1 and MRD <5% | 91.9 +/– 1.3% | p=0.41 | |
| EFS | B-ALL | M1 and MRD ≥5% | 59.1 +/– 6.5% | M1 and MRD <5% | 87.1 +/– 0.4% | p<0.0001 |
| B-ALL | M1 and MRD ≥5% | 59.1 +/– 6.5% | M2/M3 and MRD ≥5% | 39.1 +/– 7.9% | p=0.009 | |
| T-ALL | M1 and MRD ≥5% | 80.3 +/– 7.3% | M1 and MRD <5% | 87.6 +/– 1.5% | p=0.01 | |
| T-ALL | M1 and MRD ≥5% | 80.3 +/– 7.3% | M2/M3 and MRD ≥5% | 62.7 +/– 13.5% | p=0.13 |
| OS | B-ALL | M2/M3 and MRD <5% | 72.7 +/– 9.8% | M1 and MRD <5% | 93.8 +/– 0.3% | p<0.0001 |
| T-ALL | M2/M3 and MRD <5% | 100 | M1 and MRD <5% | 91.9 +/– 1.3% | p=0.41 | |
| EFS | B-ALL | M1 and MRD ≥5% | 59.1 +/– 6.5% | M1 and MRD <5% | 87.1 +/– 0.4% | p<0.0001 |
| B-ALL | M1 and MRD ≥5% | 59.1 +/– 6.5% | M2/M3 and MRD ≥5% | 39.1 +/– 7.9% | p=0.009 | |
| T-ALL | M1 and MRD ≥5% | 80.3 +/– 7.3% | M1 and MRD <5% | 87.6 +/– 1.5% | p=0.01 | |
| T-ALL | M1 and MRD ≥5% | 80.3 +/– 7.3% | M2/M3 and MRD ≥5% | 62.7 +/– 13.5% | p=0.13 |
Abbreviations: B-ALL, B-precursor acute lymphoblastic leukemia; EFS, event-free survival; OS; overall survival; MRD, minimal residual disease; T-ALL T-precursor acute lymphoblastic leukemia.
Patient groups: M1, <5% lymphoblasts; M2, 5%-25% lymphoblasts; M3, >25% lymphoblasts.
Patients with B-ALL in the M1/high MRD group had significantly inferior outcomes (5-year event-free survival [EFS]) compared with patients who were concordantly in remission; however, they had superior outcomes compared with those not in remission by morphology or MRD (Table). Of note, the average MRD levels were higher in the M2/high MRD group compared with M1/high MRD patients. Patients with T-ALL in the M1/high MRD group also had inferior five-year EFS compared with patients concordantly in remission, but their outcomes were not statistically different compared to M2/high MRD patients (Table).
In Brief
In summary, Dr. Gupta and colleagues confirmed the results from the UKALL 2003 study that suggest patients in morphologic remission but with high MRD have inferior outcomes. The authors recommend that the definition of remission be modified to incorporate flow cytometry. Some issues do arise when considering a change in the definition of remission. First, flow cytometry requires human interpretation, and MRD quantification may not be as accurate when tested outside of a few central laboratories. Second, immunotherapies, which are now used more commonly in the treatment of ALL, can alter surface immunophenotype and impact the interpretation of flow-based MRD. Third, failure to achieve remission is counted as an event in some clinical trials. Changing the definition of events will impact the comparison of outcomes across trials. Finally, the definition of relapse is arguably tied to the definition of remission, and changing the definition of relapse also raises many considerations. For example, historically the duration of first remission is used to decide whether a child with relapsed ALL should undergo HSCT in second remission. These minor and circumventable caveats aside, the data from the COG and MRC provide a compelling rationale that strongly suggests it is time to change the decades-old definition of ALL remission.
References
Competing Interests
Dr. Teachey indicated no relevant conflicts of interest.