Editor's Note
The opinions expressed by the author are his own and do not necessarily reflect those of the U.S. Department of Health & Human Services Advisory Committee on Blood and Tissue Safety and Availability, for which he is the current chair.
Transfusions save lives, and many in our communities roll up their sleeves to donate blood and platelets. Patients benefit from these altruistically motivated donations when prescribed appropriately. Randomized controlled trial results, clinical guidelines, patient blood management, and Choosing Wisely programs demonstrate favorable outcomes when patients receive fewer transfusions than given a decade ago.1,2
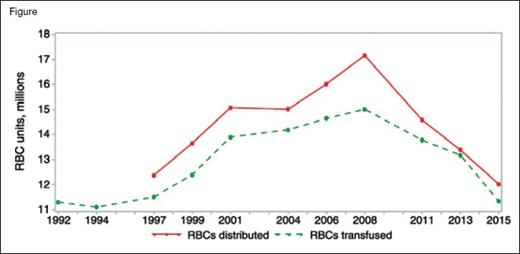
Red cell collections and transfusions peaked in 2008 at approximately 17,286,000 units collected, 17,159,000 units distributed, and 15,014,000 units transfused. By 2015, collections, distributions, and transfusions declined dramatically, by 12,591,000, 12,028,000, and 11,349,000 units, respectively. Note the narrowing of the distribution and transfusion curves, possibly representing diminished resiliency and surge capacity. Reprinted by permission from John Wiley & Sons, Ltd: Transfusion doi: 10.1111/trf.14165, copyright 2017.
Red cell collections and transfusions peaked in 2008 at approximately 17,286,000 units collected, 17,159,000 units distributed, and 15,014,000 units transfused. By 2015, collections, distributions, and transfusions declined dramatically, by 12,591,000, 12,028,000, and 11,349,000 units, respectively. Note the narrowing of the distribution and transfusion curves, possibly representing diminished resiliency and surge capacity. Reprinted by permission from John Wiley & Sons, Ltd: Transfusion doi: 10.1111/trf.14165, copyright 2017.
Blood collection and red cell transfusions peaked during 2008.3,4 By 2011, the proportion of hospitalized patients receiving transfusions plummeted.5 In 2015 (the most recent year for which comprehensive data are available), collections and red cell transfusions declined by 27.2 percent and 24.4 percent, respectively, compared to 2008 levels (Figure). Some reports indicate additional utilization decreases of 2.2 percent and 4.38 percent in 2016 and 2017, respectively, with expectations for continued contraction. Consequently, hospitals have achieved significant cost savings. Assuming a blood center charge of $200.00 per red cell unit and a decline of 3.67 million red cell transfused units between 2008 and 2015, hospital costs (and blood center revenues) declined by more than $700 million in 2015 alone.
Due to long-standing payment and coverage practices, there is no direct link between hospital reimbursement and payment to blood centers. Hospitals recover the costs for blood products through bundled payment reimbursement from insurers or the U.S. Centers for Medicare & Medicaid Services (CMS), not a per-unit charge. In turn, hospitals pay blood centers for blood products through negotiated contracts. For years, this arrangement permitted blood centers to recover costs and undertake improvements, but it faltered following hospital consolidations, mergers, and acquisitions that shifted the negotiating power from smaller regional blood centers to larger hospital groups. Blood centers also consolidated, merged, or aligned with group purchasing organizations to achieve efficiencies and reduce costs. However, significant infrastructure expenses remained, resulting in blood center budgetary shortfalls. Larger blood centers sought a greater proportion of a dwindling market by offering lower prices to hospitals. Blood charges declined, blood centers distributed fewer units, the blood supply system faced financial uncertainty, and blood centers were threatened with possible closures.
In September 2015, the U.S. Department of Health & Human Services (HHS) contracted the RAND Corporation to assess blood supply system instability. The HHS Advisory Committee on Blood and Tissue Safety and Availability (ACBTSA) provided guidance for reviewing the report. In November 2016, RAND presented its findings and recommendations in a report titled, “Toward a Sustainable Blood Supply in the United States.”6 Represented predominantly through an economic lens and based on limited data, RAND’s conclusion was that the system functioned effectively through these challenges to date. They also conceded that continued stress on blood centers could lead those centers to reduce their investments in research, innovation, and surge capacity, causing shortages, especially during emergencies. RAND saw price competition between blood centers as an incentive for reducing fixed costs (i.e., fewer blood centers) and indicated that the current system was not conducive to private investments in innovation and suppliers to blood centers face significant uncertainty.”
RAND recommended “targeted policy intervention,” collecting more data about blood use and financial arrangements, developing a vision for appropriate surge capacity levels and emergency response plans, paying blood centers for maintaining surge capacity, and paying directly for new technologies where there is no private business case for adoption. Subsequently available data indicate that more than 90 percent of blood providers’ expenses exceeded revenues in 2016.7
In its more global analysis, ACBTSA thought that RAND overstated the economic benefits of competition and price reductions while underestimating risks to the blood system. In its report to the Assistant Secretary for Health, ACBTSA stated, “Blood is a public good, built on the altruism of non-remunerated blood donors. Simple supply and demand economic principles do not fully address the societal value of this critical national resource … There is an urgency that requires a near-term solution — the resiliency of the blood system is at risk.”8 ACBTSA recommended that the U.S. government explore direct payments to blood centers for the infrastructure costs of maintaining adequate blood supplies for the public good and examine future policy making that includes all stakeholders in the vein-to-vein process from blood donor recruitment to bedside infusion.
Action on these recommendations remains in abeyance pending additional data collection in support of the tweaks suggested by RAND, or the more “crisis”-oriented interventions suggested by ACBTSA and a recent New England Journal of Medicine article.7 That article, authored by seasoned, senior transfusion medicine leaders, eloquently describes these events and provides supplementary background information. The authors conclude that “allowing [the U.S. blood] system to function as it has while it is losing stability, resilience, and surge capacity is not a responsible option,” and caution that “a constructive intervention to stabilize the U.S. blood system, although urgently needed, has yet to be envisioned.” The importance of addressing these issues stems from the reliance of hematologists/oncologists on the blood system for a safe and adequate blood supply, resolving complex red cell and platelet immunohematology problems, and providing specialized transfusion products. Hematology/oncology patients benefit from improvements in red cell and platelet transfusion storage solutions, emerging infectious agent detection, information technology interfaces, and genomic antigen identification. In one report, 56 percent of hospitalized patients with blood disorders received red cell transfusions, and 15 percent received platelet transfusions.9
To date, blood collection agencies retain the ability and capacity to respond rapidly to emerging infectious disease threats, needs for mass shooting victims, and supply disruptions caused by major weather disturbances. In Spring 2016, U.S. blood centers provided sufficient excess blood collections to Puerto Rico, where blood collection ceased during a Zika epidemic.10,11
However, challenges threaten this resiliency. Blood collectors anticipate a 4 percent decrease in blood collection following ongoing efforts to mitigate donation-associated iron deficiency among 16- and 17-year-old blood donors (250-mg Fe loss per donation). Seasonal shortages could become more generalized. Replacing lost blood donations requires additional donor recruitment efforts and added expense. As an analogy, recent saline supply shortages elucidate difficulties with ramping production capacity and maintaining redundancy.12 Hospitals also face decisions about pathogen reduction technology-treated platelets, point-of-care testing, or alternative strategies to reduce platelet transfusion-associated sepsis (bacteria detected in 1:4,000-1:9,000 apheresis platelets; reported platelet-associated sepsis in 1:108,000).13 Additionally, transfusion-transmitted Babesia microti infections represent a red cell transfusion hazard. Testing for this agent represents another decision point.14
The obstacles described in this article represent possible cost increases for hospitals or additional financial pressures for blood centers if they absorb the associated costs. Although recent observations indicate that total blood center revenues (including gains from reserve account investments) exceeded expenses by approximately 1.7 percent during 2017,10,11 a more granular assessment reveals that aggregated operating expenses exceeded revenues. Hospitals face cost-containment restraints, and insurers and CMS seek reimbursement reductions. As such, it is obligatory that informed hematologists/oncologists and their transfusion medicine colleagues become actively involved and advocate for a sustained, safe, and adequate blood system.
References
Competing Interests
Dr. Menitove indicated no relevant conflicts of interest.