Last year brought the approval of two chimeric antigen receptor (CAR) T cell products for treating pediatric and young adult B cell acute lymphoblastic leukemia (B-ALL) and adult diffuse large B cell lymphoma (DLBCL): tisagenlecleucel and axicabtagene ciloleucel, respectively. These approvals were based on the remarkably high overall response rates and highly durable complete responses seen in highly refractory and multiply relapsed disease on the ELIANA and ZUMA-1 clinical trials.1,2 The result was the establishment of a highly effective standard-of-care therapy for patients who previously did not have one, and potential curative therapy for a significant subset of patients. While revolutionary, these therapies do leave plenty of room for improvement, ranging from the identification of mechanisms of both innate and acquired resistance, to a better understanding of the mechanisms and management of cytokine release syndrome and neurologic toxicity, to the establishment of more efficient, and quicker, mechanisms of manufacturing. The last item is critical to making this therapy more widely available because the refractory and progressive high-grade B cell malignancies that these two anti-CD19 CAR T-cells treat do not pause for the necessary three- to five-week manufacturing window.
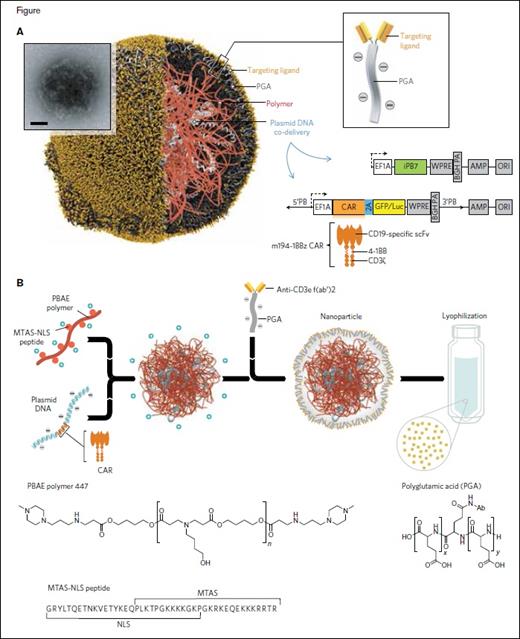
A, Schematic of the T cell-targeted DNA nanocarrier used in the authors’ experiments. The inset shows a transmission electron micrograph of a representative nanoparticle (scale bar, 100 nm). Also depicted are the two plasmids that were encapsulated into the nanoparticles; these encode an all-murine 194-1BBz chimeric antigen receptor (CAR) and the hyperactive iPB7 transposase. EF1A, eukaryotic translation elongation factor 1 alpha 1; BGH PA, bovine growth hormone polyadenylation signal; ampicillin resistance gene; ORI, origin of replication. B, Diagram describing the fabrication of the poly(β-amino ester) nanoparticles. Also shown are the chemical structures of the PBAE 447 polymer and polyglutamic acid, as well as the amino acid sequence of the microtubule-associated-nuclear localization (MTAS-NLS) peptide.
A, Schematic of the T cell-targeted DNA nanocarrier used in the authors’ experiments. The inset shows a transmission electron micrograph of a representative nanoparticle (scale bar, 100 nm). Also depicted are the two plasmids that were encapsulated into the nanoparticles; these encode an all-murine 194-1BBz chimeric antigen receptor (CAR) and the hyperactive iPB7 transposase. EF1A, eukaryotic translation elongation factor 1 alpha 1; BGH PA, bovine growth hormone polyadenylation signal; ampicillin resistance gene; ORI, origin of replication. B, Diagram describing the fabrication of the poly(β-amino ester) nanoparticles. Also shown are the chemical structures of the PBAE 447 polymer and polyglutamic acid, as well as the amino acid sequence of the microtubule-associated-nuclear localization (MTAS-NLS) peptide.
Dr. Tyrel T. Smith and colleagues report on a novel mechanism for the in vivo, and therefore nearly instantaneous, production of CAR T-cells.3 They created nanoparticles targeted to T cells by coupling poly (β-amino ester) -based nanoparticles to anti-CD3e f(ab) fragments. Upon binding to CD3 on T cells, these nanoparticles are endocytosed. Their contents, in this case a plasmid DNA encoding an anti-tumor antigen CAR, are directed to the T cell nucleus due to the inclusion of peptides containing microtubule-associated sequences (MTAS) and nuclear localization signals (NLSs). The final nuance of their manufacturing technology, namely the inclusion of transposons flanking the CAR gene expression cassette and a separate plasmid encoding a hyperactive transposase, allows for the efficient integration of the CAR vector into chromosomes (Figure 1). The result is a system that allows for the in vivo production of CAR T cells following nanoparticle infusion.
Using an anti-CD19-41BB CAR construct, they went on to demonstrate efficient nanoparticle–T cell binding within four hours of infusion in mouse models, with approximately 34 percent of CD3+ T cells bound to nanoparticles including representative T cells from all T cell subsets. Endocytosis of these particles is evident by confocal microscopy shortly thereafter. Nanoparticles can be found bound to non–T cell hematologic elements, including B cells, monocytes, and neutrophils, but in much lower numbers. In the presence of B-ALL and tumor antigen, there is a resultant proliferation of these anti-CD19 CAR T cells with high-level expression of the anti-CD19 CAR. Using a luciferase reporter, CAR T cells were visualized within the spleen by day 3 and in the bone marrow and lymph nodes shortly thereafter. CAR T cell levels peak between days 6 and 12. Both the MTAS/NLS peptides and transposon/transposase elements were necessary for these findings. In contrast to the CAR T cells that are generated, only 1 percent of off-target cell populations express the anti-CD19 CAR, and this fraction decreases throughout the first 12 days following infusion while CAR T cell levels are increasing.
In Brief
While the ability to generate CAR T cells in vivo is an elegant and incredibly innovative way to overcome the potentially rate-limiting manufacturing times needed for the current ex vivo technologies, it needs to maintain, or improve upon, the efficacy of conventional CAR T cell technologies to become a meaningful clinical therapy. Dr. Smith and colleagues were able to demonstrate antitumor efficacy in mouse models of B-ALL using their anti-CD19-41BB CAR, similar to that seen with conventional CAR T cell treatment, with both groups yielding significantly improved survivals compared with negative controls. What remains to be seen is whether this technology will be effective in generating CAR T cells in humans, whether the response rates and durability of responses will be comparable to those seen with conventional CAR T cell therapies, and whether there is an unforeseen safety issue due to off-target nanoparticle binding and CAR expression. If successful, however, in phase I clinical trials, this technology would be transformative, allowing for the immediate treatment of patients, greatly expanding the applicability of this therapy to patients in whom progression during manufacturing would otherwise be rendered too sick for eventual therapy.
References
Competing Interests
Dr. Jacobson indicated no relevant conflicts of interest.