Epigenetic aberrations are commonplace in cancer, and the past decade has seen the identification of a wide range of pathogenic variants in numerous epigenetic regulators in neoplasms, including myeloid neoplasms. Genes involved in histone modification in particular are targeted frequently by mutation. Histone modification marks include acetylation, methylation, ubiquitination, sumoylation, and phosphorylation, with enzymes that “write,” “erase,” or “read” these marks. Bromodomains are “readers” of histone acetylation of lysine tails. BRD4 is a ubiquitous member of the bromodomain and extra terminal (BET) family of bromodomain proteins and plays a key role in transcription elongation through its recruitment of P-TEFb. It targets key enhancers and superenhancers that regulate critical genes involved in tumorigenesis. Accordingly, BET inhibitors represent an enticing class of experimental anticancer drugs.
Recently, Dr. Hui Yang and colleagues created excitement in the discussion of BET inhibitors with their dramatic finding that pathogenic variants in ASXL1 result in a truncated protein that binds directly to BRD4 to induce transcription of genes that confer a hematopoietic stem-cell (HSC) advantage. Pathogenic variants in ASXL1 are seen recurrently in nearly all diagnostic categories of chronic and acute myeloid neoplasms, associated with poor prognosis.1 Additionally, these variants may also be seen in age-related clonal hematopoiesis and are associated with higher risk of progression to a subsequent myeloid neoplasm.2 All proven pathogenic variants in ASXL1 are truncating. These nonsense or frameshift variants occur in exons 11 and 12, resulting in loss of the C-terminal PHD domain (residues 1479-1539), even though the transcript is still expressed.3 Dr. Yang and colleagues have now demonstrated that the truncated protein has distinct altered function through its interaction with BRD4 from that of the wild-type protein, opening up exciting new therapeutic avenues for targeted therapy in ASXL1-mutated myeloid neoplasms.
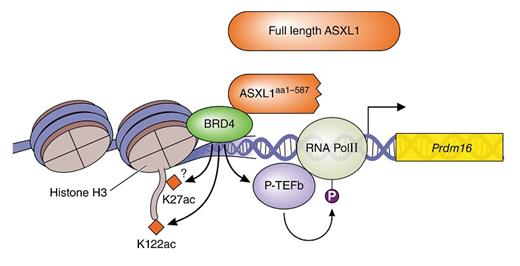
Using a transgenic mouse model engineered with an ASXL1Y588X mutation (a common recurrent nonsense mutation seen clinically), Dr. Yang and colleagues demonstrated that this truncated form can enhance the expression of key genes involved in stem-cell maintenance and myeloid differentiation. The researchers focused on Prdm16, whose gene product interacts with key developmental genes such as CCAAT/enhancer binding proteins as well as PPARG.4 Their data suggest that ASXL1 mutation plays key roles in hematopoietic colony formation and stem-cell expansion through increased transcription of Prdm16. Significantly, the truncated ASXL1, but not the WT protein, binds to BRD4 to recruit phosphorylated RNA polymerase II (RNAPII-pSer2) and acetylated H3K122 (H3K122Ac) to the promoter region of Prdm16 (Figure 1) as shown by reciprocal immunoprecipitation studies. Thus, ASXL1 truncation mutations result not in a loss of function or dominant negative function, but rather in specific gain of altered function.
This gain of altered ASXL1 function generates enhancement of the HSC pool with increases in Lin-SCA-1+cKit+ (LSK) cells, both long- and short-term HSCs, colony formation and replating ability, and the ability to reconstitute the marrow of recipient mice in transplantation studies. Additionally, differentiation of the HSCs is also affected by this ASXL1 truncation, with a shift from erythroid and megakaryocytic differentiation to increased myeloid differentiation morphologically, immunophenotypically, and in gene-expression profiling studies.
This ASXL1 truncation model mimics many of the features of a variety of myeloid neoplasms. The mice had shorter mean survival (P < 0.01), relative neutrophilia (P = 0.017), increased platelets (P = 0.048), relatively decreased lymphocytes (P = 0.005), and decreased hemoglobin and red blood cells (P = 0.004 and 0.007, respectively). In keeping with the clinical associations of ASXL1 mutations, the transgenic mice expressing the truncated protein progressed to a wide range of myeloid malignancies involving the bone marrow, blood, and spleen with features consistent with acute myeloid leukemia, myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), or MDS/MPN. Somewhat counterintuitively, based on differentiation studies in mice, the MPN and MDS/MPN cases were characterized by significant thrombocytosis with megakaryocytic hyperplasia. As megakaryocytes are known to produce a wide range of profibrotic factors, this finding is concordant with the clinical association of ASXL1 mutations with progression, including myelofibrosis, in these neoplasms.
Based on the demonstrated interaction between truncated ASXL1 and BRD4, the researchers demonstrated that ASXL1-mutant cells were specifically sensitive to treatment with BET inhibitors and that BET inhibitors repressed colony formation in a dose-dependent manner. The specificity of the inhibition was demonstrated through loss of occupancy of truncated ASXL1, BRD4, H3K122Ac, and RNAPII-pSer2 at the Prdm16 promoter.
In Brief
In summary, Dr. Yang and colleagues have provided the critical link between ASXL1 truncation mutations and a novel therapeutic avenue employing BET bromodomain inhibitors through their demonstration of gain of altered ASXL1 function. As ASXL1 mutations are amongst the most common alterations seen throughout all chronic and acute myeloid neoplasms, these discoveries are especially impactful. This work also points to the potential of synergistic targeted therapies as downstream interactions such as with PPARG. With this single study, ASXL1 biology has gone from a phenomenological association with myeloid neoplasms to a mechanistic understanding that points toward the potential of directed targeted therapy for a large number of patients with otherwise poor prognosis.
References
Competing Interests
Dr. Kim indicated no relevant conflicts of interest.