Hemophagocytic lymphohistiocytosis (HLH), a striking inflammatory disorder familiar to hematologists, was originally defined in many patients as an idiopathic syndrome. It is now recognized as a primary immune regulatory disorder.1 Standard treatment for this condition, consisting of etoposide and corticosteroids, was empirically derived decades ago and has changed little since.2 However, recent translational efforts — capitalizing on insights from animal models — hint at a future where HLH may be treatable with targeted therapies rather than with conventional agents.
Variations and Complexities
Decades of research into “classic” familial or “primary” HLH has revealed that the disease is owing to genetic defects that hinder perforin-dependent lymphocyte cytotoxic function. These defects in T and natural killer cells impair an important feedback loop that regulates acute T cell activation.3,4 Primary HLH typically arises during infancy and is invariably fatal if not effectively treated. A substantial fraction of patients, particularly those presenting beyond infancy, lacks a clearly causative mutation in the perforin pathway, though mild lesions in this pathway are increasingly recognized into adulthood.5 Most older patients have obvious environmental associations or systemic triggers for HLH, including severe infection, malignancy (particularly lymphoma), or rheumatologic disorders (e.g., juvenile idiopathic arthritis). Some patients may have diverse genetic causes including inflammasomopathies or even primary immune deficiencies; however, the striking similarities between “syndromic” HLH and primary HLH suggest a common pathophysiology despite varied upstream etiologies. These similarities also suggest that iatrogenic cytokine release syndromes after immune checkpoint blockade, synthetic T cell engagers, or chimeric antigen receptor (CAR) T cell therapies, may share important commonalities with this disease of acute T cell activation.
Conventional Treatment of HLH
Conventional (i.e., historic) treatment for patients with HLH consists of chemotherapy and immunotherapy (etoposide, corticosteroids, cyclosporin A, and sometimes intrathecal methotrexate). These regimens are typically used as a bridge to hematopoietic stem cell transplantation (HSCT). Recently reported results in a group of 369 patients treated according to the HLH-2004 study indicated that at 5.2 years of follow-up, 62 percent remained alive.2 Moreover, the chemotherapeutic agents used in this approach have significant toxicities. The limitations of conventional regimens for the treatment of HLH, along with increased understanding of key inflammatory molecules, have placed greater attention on more targeted therapies for patients with this disease.
Inflammatory Pathways in HLH
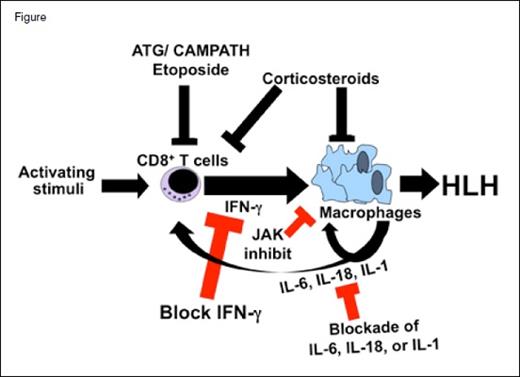
Animal models of HLH have demonstrated that it is a disease of acute T cell activation and recruitment of innate effector cells such as macrophages. Indeed, elimination of CD8+ T cells was demonstrated to be sufficient in preventing HLH development in mice6 — a finding consistent with clinical observations showing some benefit from antithymocyte globulin or alemtuzumab. Notably, though multiple inflammatory cytokines are elevated in both patients and mice with HLH, several groups have observed that interferon γ (IFNγ) is essential for disease development, which is sufficient to recreate key features of HLH in mice.6-8 While IFNγ is elevated in patients with primary HLH, it seems to be much more strikingly increased in those with EBV-associated HLH, who mostly do not have gene mutations.9-11 Elevated levels of IFNγ or CXCL9, a chemokine potently and specifically induced by IFNγ, are also observed in HLH associated with juvenile idiopathic arthritis or lymphoma.12-15 Notably, IFNγ blockade is therapeutic in diverse mouse models of rheumatologic HLH also known as macrophage activation syndrome (MAS).16,17
Evidence also suggests a contribution of other inflammatory mediators in some experimental or clinical situations. High IL-6 levels have been demonstrated to have a poor prognostic significance in HLH, and IL-6 blockade has been used to successfully treat cytokine release syndrome (a close relative of HLH) after immunotherapy for cancer.10,18 IL-18 is elevated in some patients with HLH, particularly those with genetic mutations affecting the inflammasome, and blockade is therapeutic in experimental models.19,20 Furthermore, IL-1 blockade has been reported to be therapeutic in patients with MAS.21
Hemophagocytic lymphohistiocytosis (HLH) is a disorder of excessive activation of T cells (most likely CD8+ T cells) that recruit and activate other immune effector cells, such as macrophages, to produce the clinical features of HLH. Interferon gamma (IFNγ) plays a central role in this pathologic inflammation, while other cytokines such as IL-6, IL-18, and IL-1 may contribute in certain situations or patients. Conventional therapy relies on etoposide, or sometimes anti–T cell antibodies (ATG or alemtuzumab), which deplete T cells globally (serotherapy) or target activated ones (etoposide). Corticosteroids, which act broadly against many aspects of inflammation, are also an important part of standard therapy. Targeted therapies in development for HLH target specific inflammatory mediators such as IFNγ, IL-6, IL-18, or IL-1. Signaling molecules engaged by some of these cytokines, including Janus kinases (JAKs), are being targeted as well.
Hemophagocytic lymphohistiocytosis (HLH) is a disorder of excessive activation of T cells (most likely CD8+ T cells) that recruit and activate other immune effector cells, such as macrophages, to produce the clinical features of HLH. Interferon gamma (IFNγ) plays a central role in this pathologic inflammation, while other cytokines such as IL-6, IL-18, and IL-1 may contribute in certain situations or patients. Conventional therapy relies on etoposide, or sometimes anti–T cell antibodies (ATG or alemtuzumab), which deplete T cells globally (serotherapy) or target activated ones (etoposide). Corticosteroids, which act broadly against many aspects of inflammation, are also an important part of standard therapy. Targeted therapies in development for HLH target specific inflammatory mediators such as IFNγ, IL-6, IL-18, or IL-1. Signaling molecules engaged by some of these cytokines, including Janus kinases (JAKs), are being targeted as well.
Targeted Therapy for HLH
Several approaches to targeted therapy for HLH are currently in clinical testing, and further studies are likely (Figure).
Targeting IFNγ. Emapalumab is a monoclonal antibody directed against IFNγ that is being developed specifically for the treatment of HLH. An international phase II/III clinical trial is currently ongoing (NCT01818492), though an interim analysis of results was reported at the 2015 ASH Annual Meeting.22 In this study, emapalumab was initially administered at a dose of 1 mg/kg, along with dexamethasone. Of 16 patients enrolled (median age, 1.2 years), 14 received emapalumab as second-line treatment after failure or intolerance of conventional therapy. Of the 13 patients who were evaluable and who completed treatment, nine achieved a satisfactory response: Seven proceeded to HSCT, and two were awaiting transplantation with good control of HLH. Four patients had an insufficient response, two of whom died of HLH, and two were able to proceed to HSCT. IFNγ neutralization was demonstrated in most patients by a sharp decrease in serum CXCL9. Overall, emapalumab was well tolerated with no significant safety concerns identified. This study continues to accrue patients, and development of emapalumab also continues, with open studies examining its effectiveness in secondary HLH associated with juvenile idiopathic arthritis (NCT03311854) and an observational study gathering data about IFN-γ in patients with malignancy-associated HLH (NCT03259230).
Targeting Janus kinase (JAK). IFNγ signaling proceeds via the JAK/STAT pathway, making JAK inhibition an obvious therapeutic target. Ruxolitinib, a U.S. Food and Drug Administration–approved inhibitor of JAK1 and JAK2 can block signaling downstream of IFNγ, IL-6, IL-10, and other cytokines. In preclinical murine models, treatment with ruxolitinib has been reported to be therapeutic, improving inflammatory pathologies such as weight loss, organomegaly, cytopenias, and tissue infiltration, as well as improving survival.23,24 Two individual case reports of patients with syndromic or secondary HLH treated with ruxolitinib have been published recently.25,26 Both patients displayed decreases of inflammatory markers, though one did not experience recovery of cytopenias and died. The potential myelosuppressive effects of ruxolitinib will warrant attention in future clinical studies in this disease. A phase I trial evaluating ruxolitinib for patients with HLH is now recruiting (NCT02400463).
Targeting CD52. Alemtuzumab is a humanized monoclonal antibody directed against CD52, which depletes T cells, B cells, and monocytes. A retrospective report describing treatment with alemtuzumab in 22 patients with refractory HLH indicated that approximately two-thirds of patients experienced an overall partial response and 77 percent of patients survived to undergo HSCT.27 Use of alemtuzumab is associated with profound and long-lasting immune suppression, though in this report, the risk seemed justified by the observed benefit. Alemtuzumab is currently being evaluated in a clinical trial of patients with primary HLH (NCT02472054). A second trial is examining alemtuzumab in combination with conventional agents for the treatment of malignancy-associated HLH (NCT02385110).
Targeting IL-6. IL-6 is an inflammatory cytokine that is typically elevated in patients with HLH and has been linked to cytokine release syndrome.11,18 Results from case studies have suggested that tocilizumab, a humanized anti–IL-6 receptor monoclonal antibody, may be effective in the treatment of HLH and cytokine release syndrome.28,29 Notably, chronic treatment with tocilizumab did not prevent the development of HLH (MAS) in patients with juvenile idiopathic arthritis.30 Two clinical trials of tocilizumab in patients with HLH are in progress. One is open to both pediatric and adult patients with HLH (NCT02007239), and the other is assessing treatment of malignancy-associated HLH in adults (NCT02385110).
Targeting IL-1 and IL-18. IL-18 is significantly elevated in patients with activating lesions of the inflammasome. Patients with at least two genetically defined inflammasomopathies (mutations in XIAP or NLRC4) are prone to develop HLH. A clinical trial testing the effects of recombinant IL-18 binding protein (tadekinig α) is currently enrolling patients with these genetic lesions (NCT03113760). Finally, blockade of IL-1 with IL-1 receptor antagonist (anakinra) has been reported to be therapeutic for patients with HLH (or MAS) associated with juvenile idiopathic arthritis. A clinical trial is currently enrolling these patients (NCT02780583).
Lessons Learned and Future Prospects
The development thus far of targeted therapies for HLH has illustrated two key lessons: First, animal studies have unparalleled power to define causality in disease states. It is the detailed understanding of inflammatory pathways and identification of optimal targets for intervention in these models that have allowed for development of new therapies for patients. Second, even well-studied processes or molecules can have unexpected effects in pathologic contexts. Initial understanding of the perforin pathway suggested only a role in host defense, rather than the dominant role it plays in immune regulation in humans. Furthermore, before HLH was modeled in mice, few would have predicted the unusual immunopathologies that IFNγ can produce. Current translational efforts are just beginning to uncover complexities of human HLH that animal models may not have revealed. There is much to learn and perhaps more surprises to be had as therapy for HLH begins to focus on rationally derived targets.
References
Competing Interests
Dr. Jordan serves on a scientific advisory committee for Novimmune.