Modern civilization is the result of our ability to master agriculture. As populations grew, a continual food supply cultivated from seeds was a necessity that allowed communities to expand and other endeavors to be pursued. Arguably, agriculture may be our greatest achievement.
In many ways, hematology is in the early stages of its own agricultural revolution with current efforts to grow red blood cells, platelets, cancer-killing T-cells, and myriad other cell and tissue types. Much like feeding a growing population, the goal of these farming efforts is to have a reliable source of life-saving cells to treat every patient who comes through the door. For diseases that can be treated with bone marrow transplantation, the aim is to cultivate and grow enough hematopoietic stem cells (HSCs). Current efforts in HSC expansion have shown some promise, but healthy stem cells to “seed” this farming effort are required. For instance, HSCs from a matched donor cord blood unit can be cultivated and grown to sufficient numbers to allow for successful engraftment in a patient. However, this seed source is still somewhat limited and the allogeneic nature of these expansion efforts is not ideal for many diseases. In some cases, a patient’s own cells could be used to seed the culture, but the underlying genetic cause of the disease or subsequent genetic hits may taint those stem cells as a starting material for farming efforts.
In 2006, Dr. Shinya Yamanaka reintroduced four transcription factors (OCT4, SOX2, KLF4, and MYC) into adult mouse fibroblasts that could revert the cells to an “induced pluripotent state” (iPS) capable of re-differentiating into multiple cell types.1 This discovery concretized the ability to generate patient-specific stem cells. Since then, several groups have tried to use this new seed source, iPS cells or embryonic stem (ES) cells, to generate HSCs for transplantation.2
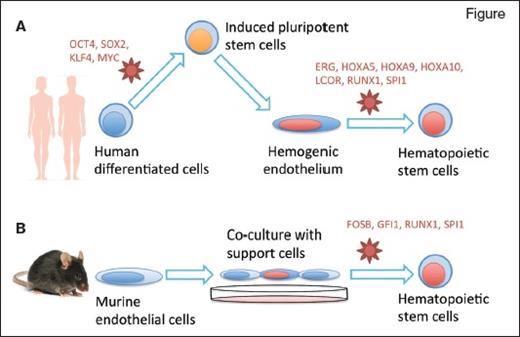
Two research groups reprogrammed differentiated cells into hematopoietic stem cells (HSCs) using specific culture conditions and retroviral delivery of transcription factors (in red). Dr. Sugimura and colleagues (A) induced pluripotency in human mature cells then redirected their differentiation into HSCs, while Dr. Lis and colleagues (B) directly reprogrammed murine endothelial cells into HSCs.
Two research groups reprogrammed differentiated cells into hematopoietic stem cells (HSCs) using specific culture conditions and retroviral delivery of transcription factors (in red). Dr. Sugimura and colleagues (A) induced pluripotency in human mature cells then redirected their differentiation into HSCs, while Dr. Lis and colleagues (B) directly reprogrammed murine endothelial cells into HSCs.
Inducing iPS or ES cells to differentiate toward a hematopoietic fate requires the cells to engage a specific epigenetic landscape, and the factors involved are not completely elucidated. Prior attempts using human or mouse pluripotent cells as a starting material could only produce progenitors with limited engraftment, short lifespan, or little T-cell differentiation potential.3,4 Two reports published at the same time by the groups of Drs. George Daley and Shahin Rafii outline new farming approaches to produce bona fide HSCs (Figure).
Dr. Ryohichi Sugimura and colleagues combined two previous methodologies. First, they used morphogens to direct differentiation of human pluripotent stem cells (both ES and iPS cells) into an embryonic tissue called hemogenic endothelium. This tissue eventually gives rise to blood stem cells in vivo, though the transition to HSCs in vitro had never been achieved previously.
Next, they screened 26 candidate hematopoiesis-specific transcription factors to further promote reprogramming of the iPS-induced hemogenic endothelium into a blood–stem-cell state. They recovered seven key transcription factors (ERG, HOXA5, HOXA9, HOXA10, LCOR, RUNX1, and SPI1) that are necessary and sufficient to convert hemogenic endothelium into functional hematopoietic stem and progenitor cells. Cells transduced with these factors could successfully produce multiple lineages of blood cells in the bone marrow and peripheral blood circulation when implanted into immunodeficient mice. Some mice were able to mount a human immune response after vaccination. Human cells that engrafted in these mice could also be transplanted in secondary recipients. Overall, the combined approach of chemically driven differentiation followed by transcription factor–mediated cell fate conversion produced functional hematopoietic stem and progenitor cells from human pluripotent stem cells.
Dr. Raphael Lis and colleagues took a different approach to generate HSCs. Since HSCs share a common ancestor with endothelial cells in the embryo (the hemogenic endothelium), the authors hypothesized that it might be possible to switch the fate of endothelial cells into HSCs directly — a transdifferentiation approach. To achieve this, they used a combination of a co-culture system and ectopic expression of transcription factors. Endothelial cells purified from adult mice were co-cultured with a supportive cell line, which the authors had previously shown could expand HSCs in vitro, consisting of human endothelial cells in which the Akt pathway is constitutively activated.5 The murine endothelial cells were transduced with four transcription factors (FOSB, GFI1, RUNX1, and SPI1) under the control of a doxycycline-inducible promoter. Administration of dox in culture allowed the cells to transdifferentiate and expand, then dox was removed and the cells were transplanted in mice. The result was multi-lineage engraftment, including functional T-cell compartments, and bona fide HSCs as evidenced by secondary transplantation.
In Brief
For the moment, these results are mostly a proof of the feasibility of HSC generation from this alternative seed source, but they are a tantalizing breakthrough. Starting from induced pluripotent cells (Sugimura et al) or endothelial cells (Lis et al), two groups could generate hematopoietic cells with multilineage reconstitution potential in vivo, including particular functional T-cell progeny, which had eluded previous efforts. This lays a framework to study the ontogeny of HSCs in a controlled environment and identify novel regulators. Many areas remain to be improved: The HSCs generated by Dr. Sugimura and colleagues did not engraft as well as normal adult human HSCs, and the work of Dr. Lis and colleagues was entirely done in mouse cells. Regarding clinical applications, the absolute number of HSCs generated in these experiments is several orders of magnitude lower than what is required to transplant in a human. Additionally, both approaches relied on lentiviruses to introduce extra copies of transcription factors into the genome of the cells. Some of these genes are also linked to leukemia, and thus, the risk of malignant transformation associated with this approach should be carefully assessed. A reprogramming approach entirely induced by small molecules would be more desirable in that regard. With the expansion of diseases that can be treated by bone marrow transplantation, coupled with the increase in potential patients, a continual supply of high-quality HSCs will be needed. A stem cell agricultural revolution may ultimately meet this demand.
References
Competing Interests
Dr. Mercier, Dr. Rhee, and Dr. Hoggatt indicated no relevant conflicts of interest.