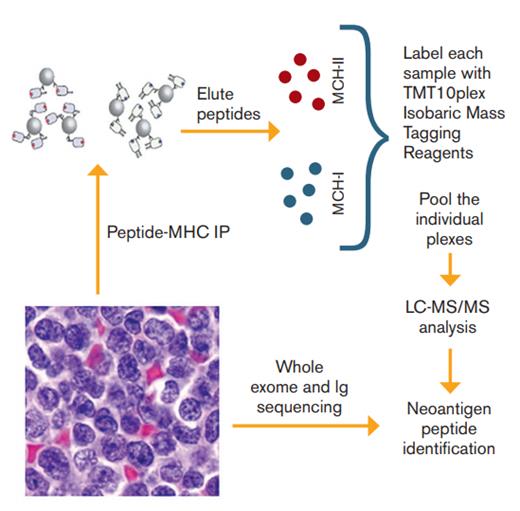
Schematic of Proteomic and Sequencing Platform Used to Identify Tumor Neoantigens. Tumor samples from 17 patients with mantle cell lymphoma were used to 1) isolate major histocompatibility complex (MHC) ligands through immunoprecipitation (IP) followed by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) and 2) perform whole exome sequencing and immunoglobulin gene sequencing in order to identify neoantigen peptides.
Schematic of Proteomic and Sequencing Platform Used to Identify Tumor Neoantigens. Tumor samples from 17 patients with mantle cell lymphoma were used to 1) isolate major histocompatibility complex (MHC) ligands through immunoprecipitation (IP) followed by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) and 2) perform whole exome sequencing and immunoglobulin gene sequencing in order to identify neoantigen peptides.
Dr. Michael Khodadoust and colleagues use liquid chromatography and tandem mass spectrometry to perform direct proteomic analysis of 17 primary mantle cell lymphoma (MCL) samples and two MCL cell lines to identify major histocompatibility complex– (MHC-) presented tumor neoantigens. Through immunoprecipitation, they isolated 24,000 unique MHC-I–associated peptides and 12,500 unique MHC-II-associated peptides. Combining this with whole-exome sequencing and direct sequencing of immunoglobulin (Ig) heavy- and light-chain–variable regions, the researchers found that of the approximately 13 to 175 nonsynonymous somatic mutations per patient, only mutated peptides derived from Ig genes were presented by MHC. For all others, only unmutated parts of the protein were presented. Interestingly, MHC presentation was polarized such that nearly all Ig-variable neoantigens were presented by MHC-II, whereas the majority of Ig-constant neoantigens were presented by MHC-I. Of the Ig-variable neoantigens, just about half of them were the result of somatic hypermutation or V-D-J recombination.
Using synthetic neoantigen peptide tetramers with affinity for HLA-DR*0401, they screened the blood of three patients with an HLA-DR*0401 allele for neoantigen-specific CD4+ T cells and found them in one of the three patients. These CD4+ T cells appeared to be memory T cells and lacked PD-1 expression, and were skewed towards a Th2/Th17 phenotype. T-cell receptor (TCR) sequencing identified two dominant T-cell clones, both of which were induced upon neoantigen peptide autologous vaccination. Ex vivo expanded neoantigen specific CD4+ T cells stimulated by autologous neoepitopes resulted in the production of IL-4 and granzyme and could mediate the killing of autologous lymphoma cells in an antigen-specific manner.
In Brief
Non-Hodgkin lymphomas (NHLs) are susceptible to immune attack, as evidenced by the efficacy allogeneic stem cell transplantation, but can evade host-immune recognition. Attempts to harness the host’s own immune system against the lymphoma with immune checkpoint blockade have been less successful than in Hodgkin lymphoma or certain solid tumors. Dr. Khodadoust and colleagues examined a panel of primary MCL samples and cell lines by direct proteomic antigen profiling to identify tumor neoantigens and their potential to elicit an antitumor immune attack. They demonstrate the feasibility of such an approach and identify genes in the Ig-variable region to be the major source of MHC-presented lymphoma neoantigens. Strikingly, these neoantigens are presented almost exclusively in the context of MHC class II, and the cognate neoantigen-specific CD4+ host T cells are skewed to a Th2 phenotype. Why or how is unclear at this point, but it may lead to tumor immune evasion, or even tumor cell progression via support and activation by cognate helper T cells. Despite this, these CD4+ T cells are able to mediate tumor cell killing in an antigen specific manner and autologous tumor vaccination results in induction of anti-tumor CD4+ T cell clones. This has therapeutic potential, especially in a group of diseases with relatively low mutation burden, which may be predicted to be less responsive to immune checkpoint blockade. Identification and ex vivo expansion of autologous neoantigen specific CD4+ T cells has the potential to be a new form of cell therapy for these patients.
Competing Interests
Dr. Jacobson indicated no relevant conflicts of interest.